Abstract
The structural complexity of the brain depends on precise molecular and cellular regulatory mechanisms orchestrated by regional morphogenetic organizers. The thalamic organizer is the zona limitans intrathalamica (ZLI), a transverse linear neuroepithelial domain in the alar plate of the diencephalon. Because of its production of Sonic hedgehog, ZLI acts as a morphogenetic signaling center. Shh is expressed early on in the prosencephalic basal plate and is then gradually activated dorsally within the ZLI. The anteroposterior positioning and the mechanism inducing Shh expression in ZLI cells are still partly unknown, being a subject of controversial interpretations. For instance, separate experimental results have suggested that juxtaposition of prechordal (rostral) and epichordal (caudal) neuroepithelium, anteroposterior encroachment of alar lunatic fringe (L-fng) expression, and/or basal Shh signaling is required for ZLI specification. Here we investigated a key role of Wnt signaling in the molecular regulation of ZLI positioning and Shh expression, using experimental embryology in ovo in the chick. Early Wnt expression in the ZLI regulates Gli3 and L-fng to generate a permissive territory in which Shh is progressively induced by planar signals of the basal plate.
Introduction
The zona limitans intrathalamica (ZLI) is a neuroepithelial domain intercalated between the prethalamus and the thalamus in the alar plate of the diencephalon (prosomeres p3 and p2, respectively; Rendahl, 1924; Puelles et al., 1987; Rubenstein et al., 1994; Shimamura et al., 1995; Martínez and Puelles, 2000). The production and release of the Shh morphogen underlies the hypothesis that the ZLI is a secondary organizer. In addition to the ZLI, other neighboring diencephalic domains release signals such as retinoid acid or members of the Fgf, Bmp, and Wnt families. They jointly control the expression of regulatory genes encoding positional information in the thalamic neurepithelium, required to develop the diencephalic structural complexity (Scholpp and Lumsden, 2010; Martinez-Ferre and Martinez, 2012).
In chick embryos, it was proposed that interaction between prechordal [Six3-positive (Six3+)] and epichordal (Irx3+) neuroepithelium regulates ZLI specification (Kobayashi et al., 2002; Vieira et al., 2005; Guinazu et al., 2007). However, Six3 is not required for the formation of the mammalian ZLI (Lavado et al., 2008). Alternatively, it also has been postulated that rostral Fez versus caudal Otx expressions regulates ZLI specification (Scholpp and Lumsden, 2010). The transverse ZLI is singular in brain regionalization, because it represents the only neural area in which Shh, normally a ventrodorsal polarizing signal (Ericson et al., 1996; Watanabe and Nakamura, 2000) regulates anteroposterior regionalization. However, the mechanisms underlying the positioning and activation of Shh in ZLI cells are still unclear. Interestingly, the intrathalamic boundary coincides with Wnt8b expression at early stages of development (Garda et al., 2002). This expression is complementary to the lunatic fringe (L-fng) expression domain, also held to be involved in ZLI formation (Zeltser et al., 2001).
Specification of cell identities in the neural tube is partly regulated by antagonistic interaction between dorsalizing and ventralizing signals, coded by Wnt (Lee and Jessell, 1999) and Shh (Jessell, 2000) signals stemming from the roof and floor plates, respectively. Such signals are reproduced and superposed at 90° in the alar diencephalon, in which Wnt8b is expressed transversally preceding Shh in the ZLI. Although some studies excluded a role of Wnt expression in Shh activation in the ZLI (Guinazu et al., 2007), we reexamined Wnt-related mechanisms controlling Shh expression in ZLI cells in chick embryos.
Our results show that inhibition of Wnt signaling in the alar diencephalon stops the dorsal progression of Shh expression in the ZLI through a Shh/Gli pathway-mediated mechanism. Gli3 is a transcriptional repressor regulated by Shh (Schimmang et al., 1992) and mediates Shh signaling (Persson et al., 2002; Abbasi et al., 2010). Our data indicate that Wnt-signal-mediated inhibition of Gli3 precedes ZLI development and generates permissive conditions for the activation of Shh at the p3/p2 boundary. A broad initial expression of Wnt8b around the prospective organizer causes heterogeneous molecular interactions with other agents of ZLI formation, and the subsequent progressive reduction of the ZLI Wnt expression into a sharp transverse stripe may represent a mechanism organizing local positional information and stabilizing molecular interactions that underlie the specification of the morphogenetic organizer.
Materials and Methods
Experimental embryology
All animal experiments were performed in compliance with the Spanish and European Union laws on animal care in experimentation (Council Directive 86/609/EEC) and have been analyzed and approved by the Animal Experimentation Committee of our university. Fertilized chick (Gallus gallus) and quail (Coturnix coturnix) eggs were incubated at 37°C in a forced-air incubator. The embryos were staged according to Hamburger and Hamilton (1951).
Implantation of microbarriers
Metal microbarriers were implanted into the right side of the neural tube as described previously (Vieira and Martinez, 2006). Microbarriers were inserted between the basal plate and the prospective ZLI (Garcia-Lopez et al., 2004). Embryos were then allowed to develop until Hamburger–Hamilton stage 23 (HH23) before overnight fixation with 4% PFA in PBS at 4°C.
Implantation of Dkk-1-soaked beads
Implantation of Dkk-1 beads into the neural tube of chick embryos was performed as described previously (Crossley et al., 1996; Vieira and Martinez 2005). Beads were implanted at the prospective ZLI at HH10. Heparin acrylic beads were rinsed in PBS and then soaked in a solution of 25 μg/ml Dkk-1 protein in PBS/0.1% BSA at 4°C overnight. Afterward, the beads were rinsed in PBS several times and then implanted into the neural tube of the embryos. For the control experiments, beads were soaked in PBS/0.1% BSA in the same manner. Embryos were fixed in 4% PFA at 4°C overnight, 24 h or 3–4 d after bead implantation.
Preparation of organotypic cultures
Chick embryos were developed until stage HH21 and prepared for organotypic culture as described previously (Echevarría et al., 2001). Briefly, chick neural tubes were dissected, telencephalic vesicles were partially removed, and neural tubes were opened through the dorsal midline and cultured ventricular side up. Implantation of beads was performed as described previously (Echevarría et al., 2001; Martinez-Ferre and Martinez, 2009). Dkk-1- or PBS-soaked beads were implanted at the dorsal end of the ZLI, visible at these stages using incident light in the central region of the diencephalic alar plate, and cultured for 24 h, before fixation.
Grafting experiments
Heterotopic and isochronic or heterochronic grafts were performed using quail embryos as the source of diencephalic or mesencephalic neuroepithelium implanted into the alar region of either the diencephalon (p1 or p2) or the mesencephalon of host chick embryos. The chimeric embryos were produced as described by Garcia-Lopez et al., (2004). After microsurgery, the eggs were sealed and incubated until stages HH23 or HH25.
In situ hybridization
After fixation, embryos were rinsed in PBT (PBS with 0.1% Tween 20), gradually dehydrated using increasing concentrations of methanol, and stored in 100% methanol at −20°C before being processed for in situ hybridization (ISH). Whole-mount ISH was performed as described previously (Shimamura et al., 1994). Digoxigenin and fluorescein-labeled RNA probes were prepared from plasmids from our laboratory collection or were kindly provided by the following laboratories: A. P. McMahon (Los Angeles, CA) (Wnt8b), C. Tabin (Boston, MA) (Gli3), and L. Puelles (Murcia, Spain) (Gbx2). ISH was performed for Shh, Fgf8, Wnt8b, Wnt1, Otx2, L-fng, Gli3, Gbx2, and Nkx2.2. RNA-labeled probes were detected with alkaline phosphatase-coupled anti-digoxigenin or anti-fluorescein antibodies (Roche Diagnostics). Nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate was used as a chromogenic substrate to detect the digoxigenin-labeled probes (Roche Diagnostics), and iodonitrotetrazolium/5-bromo-4-chloro-3-indolyl phosphatase was used for the detection of the fluorescein-labeled probes (Roche Diagnostics). After ISH, embryos were washed in PBT, photographed under a dissecting microscope (Leica), and stored at 4°C in PBT with 0.1% sodium azide.
Analysis of the chimeras
After fixation, embryos were rinsed in PBT (PBS with 0.1% Tween 20), dehydrated using various concentrations of methanol, and stored in 100% methanol at −20°C before being processed for immunostaining. After processing for ISH, whole-mount immunostaining was performed using a monoclonal anti-quail antibody (QCPN; Developmental Hybridoma Bank) to detect quail tissues. Immunofluorescence for QCPN was performed by incubating the embryos with biotinylated goat anti-mouse antibody (1:200; Vector Laboratories). The secondary antibody was followed by incubation with streptavidin conjugated with Cy3 (1:700; GE Healthcare).
WNT1-expressing cells
Cell culture.
Cells from the QT-6 cell line were cultured in DMEM (Sigma), with 8% calf serum and 2% horse serum, supplemented with 100 U/ml penicillin–streptomycin (Sigma) and 2 mm l-glutamine (Sigma).
Mammalian expression vector.
The mouse WNT1 coding region without stop codon (Clon IRAVp968F0412D; Imagenes) was subcloned into the BglII site of a pSTBlue1–mRFP1vector to obtain a WNT1–mRFP fusion protein. The cDNA fragment encoding mouse WNT1 and the mRFP1 coding region were subcloned into BamHI + NotI digested pIRES1hyg vector (Clontech) to generate pCMV–WNT1–mRFP1–IRES1hyg.
Transfection methods.
One day before transfection (with Lipofectamine 2000; Invitrogen), the QT-6 cells were seeded at a density of 0.5 × 105 cells/cm2 in multiwell (24-well) plates. The cells were incubated with DNA–lipid complexes for 4 h (following the instructions of the supplier), after which the lipofection mix was removed and replaced by fresh medium. Drug selection of stable transfectants was performed with 50–100 μg/ml hygromycin B (hyg; Calbiochem).
Graft of QT6–Wnt1 cells.
Twenty-four hours before the graft, cells were seeded (1 × 106 cells/ml standard medium) into (35 mm) bacteria plates with 1% agar. Cell aggregates were transferred and grafted into chick embryos in ovo. Operated embryos were incubated at 37°C under humidified and ventilated atmosphere until they reached E3–E4 (HH20–HH23).
Results
Shh and Gli expression in the developing diencephalon
The Shh/Gli pathway plays a major role in dorsoventral patterning of the neural tube (for review, see Jessell, 2000; Ruiz i Altaba et al., 2007). In the diencephalon, Shh is the main signal expressed in the ZLI, a transverse morphogenetic organizer controlling anteroposterior diencephalic regionalization and thalamic nuclear organization in vertebrates (Echevarría et al., 2003; Vieira et al., 2005, 2010; Vieira and Martinez, 2006; for review, see Scholpp and Lumsden, 2010).
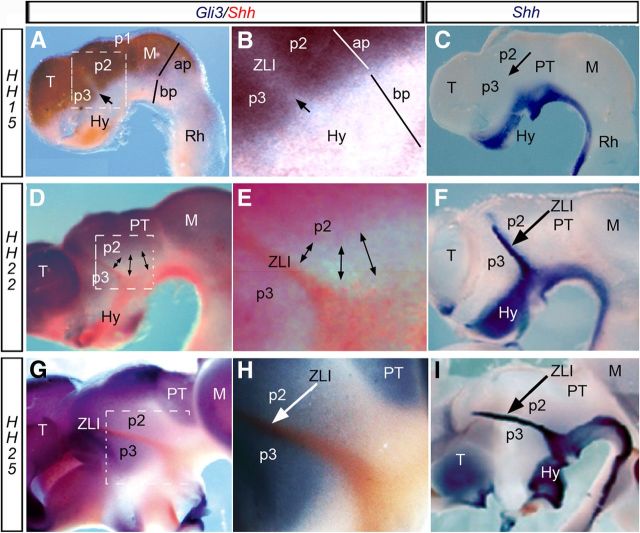
The first evidence of a ZLI in the alar diencephalic epithelium was detected at stage HH15, by the selective lack of alar Gli3 expression between p2 and p3 (Fig. 1A,B). Shh signal did not appear at the ventral edge of this ZLI primordium until stage HH17 (Garcia-Lopez et al., 2004; Kiecker and Lumsden, 2004; Vieira et al., 2005). At later stages, Shh expression expanded dorsalward into the ZLI, filling the Gli3-negative locus between p2 and p3, and reaching its dorsalmost tip between stages HH22 and HH25 (Fig. 1D–I; Vieira et al., 2005).
Figure 1.
Lateral view of the gene expression pattern at different embryological stages. A–C, HH15; D–F, HH22; G–I, HH25. A,B, D,E, and G,H show the complementary expression pattern of Gli3 (blue) and Shh (red) in the chick diencephalon. B, E, H, Higher-magnification of the squares represented in A, D, and G, respectively. Shh expression (in blue) is analyzed at HH15 (C), HH22 (F), and HH25 (I), when Fgf8 expression (red) is also detected. The arrow in A and B indicates the gap of Gli3 expression in the presumptive ZLI at HH15. Arrow in C shows the lacking of Shh in the ZLI at this stage. Double-headed arrows in D and E indicate the absence of Gli3 expression caudal to the ZLI (expressing Shh) at HH22. Arrowheads in C, F, H, and I indicate the expression of Shh in the ZLI. ap, Alar plate; bp, basal plate; Hy, hypothalamus; M, mesencephalon; PT, pretectum; Rh, rhombencephalon; T, telencephalon.
Wnt expression in the developing diencephalon
Wnt/β-catenin activity apparently controls the expression of dorsal markers and suppresses the ventral program in the whole CNS (Backman et al., 2005; Alvarez-Medina et al., 2008). Because some Wnt gene expression domains correlate with morphological boundaries in the neural tube (Hollyday et al., 1995; Quinlan et al., 2009), we analyzed the temporo-spatial expression pattern of Wnt1, Wnt2b, Wnt3a, Wnt8b, and Wnt5a genes in the chick diencephalon (Fig. 2). Among these markers, Wnt8b expression appeared early in the diencephalon (HH10) and is the only member of the family expressed at the p3/p2 boundary before and after Shh activation (Fig. 2J–L). Wnt2b and Wnt3a are both expressed in an alar p2 region from HH15 onward but not at the ZLI (Fig. 2D–F, G–I, respectively); Wnt7b is expressed in rostral prosencephalic domains after HH22 (data not shown; Garda et al., 2002), whereas Wnt5a is expressed in p2 and p3 flanking the ZLI at later stages (HH23; Fig. 2O) and Wnt1 is expressed at the dorsal midline of p1 and mesencephalon (Fig. 2A–C). Thus, although several Wnt genes expressed in neighboring regions generate Wnt signals acting on prospective diencephalic epithelium, their different expression patterns at early postneurulation stages (HH9–HH11) strongly suggest that Wnt8b would be the favorite candidate to be involved in the specification of the p3/p2 boundary (the ZLI; Fig. 2). Moreover, Wnt8b expression appears dorsally in the area of contact between a graft of rostral forebrain neuroepithelium that is apposed to caudal alar forebrain in the diencephalic alar plate at HH10 [Garda et al., 2002; Garcia-Lopez et al., 2004; these were previously conceived as prechordal grafts into epichordal domains (Vieira et al., 2005), but the whole forebrain may be seen as being epichordal; Martinez et al., 2012; Puelles et al., 2012; Fig. 3A].
Figure 2.
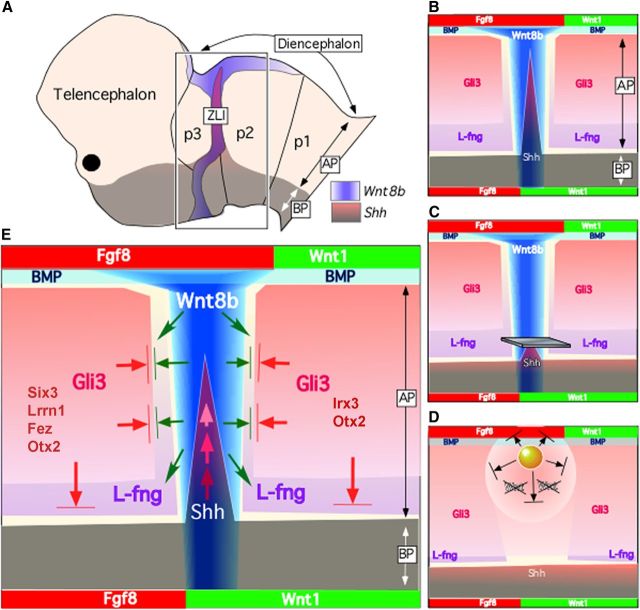
Wnt gene expression patterns in the chick prosencephalon: Wnt1 (A–C), Wnt2b (D–F), Wnt3a (G–I), Wnt8b (J–L), and Wnt5a (M–O) at HH10 (A, D, G, J, M), HH15 (B, E, H, K, N), and HH23 (C, F, I, L, O). Dorsal views (A, D, G, J, M) and lateral views (B, C, F, E, H, I, K, L, N, O) of whole-mount neural tube are shown. Dot lines mark the prospective ZLI. Green circles in A, D, G, J, and M indicate where a Dkk-1-soaked bead was implanted in bead-implanting experiments. Di, Diencephalon; Tel, telencephalon; Mes, mesencephalon.
Figure 3.
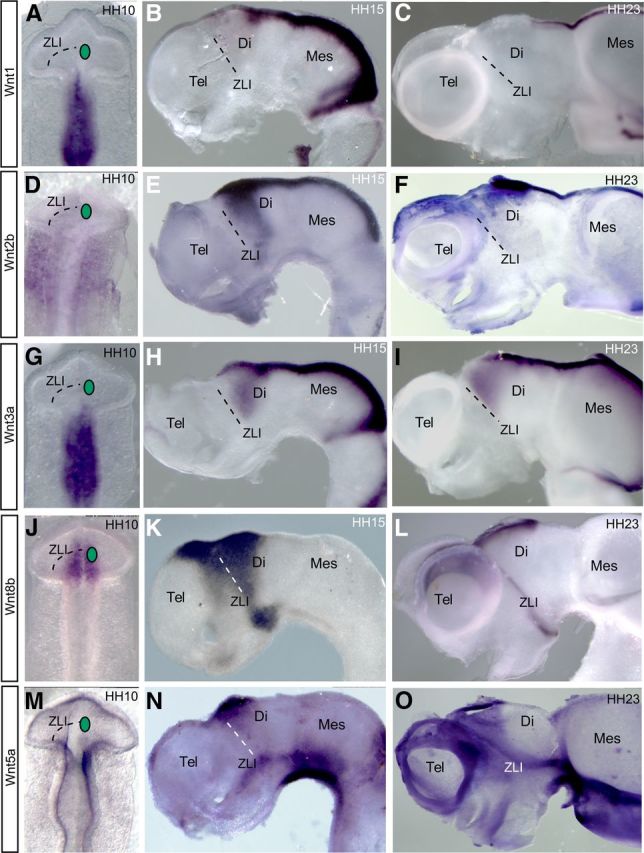
Analysis of Wnt expression at early stages of chick neural tube. A, Dorsal view of an embryo analyzed by ISH for Wnt8b expression in the diencephalon (arrow) at stage HH10. B–D, Lateral views of embryos analyzed by ISH for Wnt8b (blue) and Shh (red) at stages HH15, HH17, and HH23, respectively. B′, Homogenous pattern of Otx2 expression in the diencephalic alar plate at HH15. The arrow labels the position of ZLI. E, F, Cryostat horizontal sections (10 μm thick) of the ZLI (at the level indicated by the arrow tip) in C and D. Embryos were analyzed by ISH for Wnt8b in red and Shh in blue. G–I, Embryos analyzed by ISH for Wnt1 at stages HH10 (G) and HH22 (H, I). I, Dorsal view of an embryo analyzed by ISH for Wnt1 in the roof plate (arrows) at HH22. Arrowheads in H and I mark the rostral limit of Wnt1 expression in the diencephalon. C, Caudal; D, diencephalon; Hy, hypothalamus; M, mesencephalon; ML, mantle layer; P, prosencephalon; R, rostral; Rh, rhombencephalon; rp, roof plate; T, telencephalon; VE, ventricular epithelium.
Later, at HH15–HH17, Wnt8b expression expands transversally ventralward along the p3/p2 limit, where it meets separate expression in the basal plate of p3 and hypothalamus; it also expands extensively along the diencephalic and telencephalic dorsal midline (Fig. 3B,C). At these stages, Otx2 was homogeneously expressed in the diencephalic alar plate (Fig. 3B′). Later in development (HH23), Wnt8b expression remained strong in the telencephalic and diencephalic roof plate, whereas it was partially downregulated in the alar diencephalon, resulting in being restricted to the ZLI (Fig. 3D). Wnt1 expression was mapped at the dorsal p1 midline and extends from there backward (Fig. 3G–I). At this stage, Wnt8b and Shh were coexpressed in the ZLI cells (Fig. 3E,F), flanked by prethalamic and thalamic neuroepithelial domains showing Gli3 and L-fng expression (Fig. 1B,C,E,F; see Fig. 8A).
Figure 8.
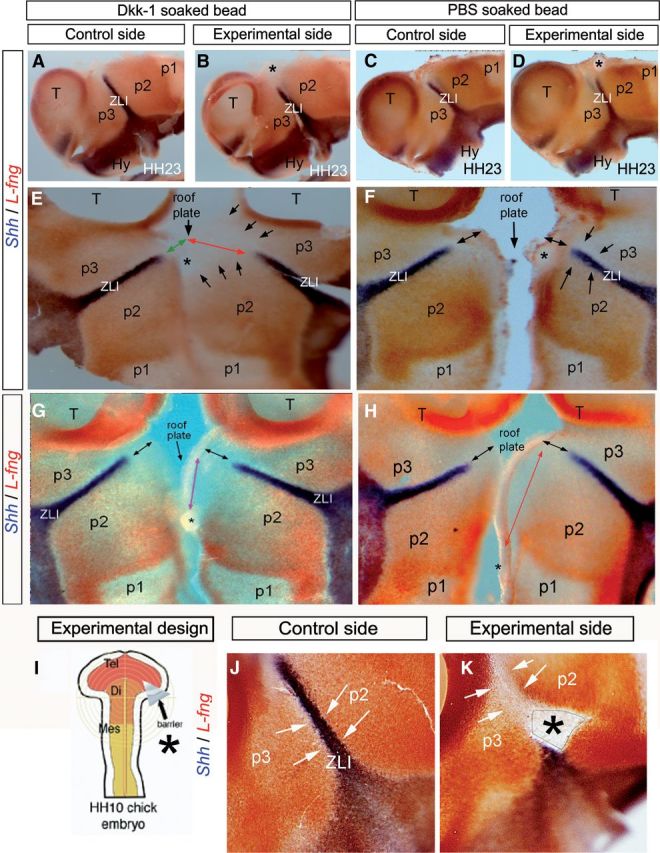
Wnt signaling is required for L-fng encroachment in the alar diencephalon. A–D, J, K, Lateral views of the whole-mount prosencephalic neural tube stained for Shh (blue) and L-fng (red) by double ISH at HH23. A, C, J, Control side of the experimental embryos. B, D, K, Experimental side of the embryos. E–H, Flat mounts opened through the ventral midline. Asterisk in B, E, and G indicates the region in which a Dkk-1-soaked bead was implanted at HH10. Asterisk in D, F, and H indicates the region in which the PBS-soaked bead was implanted. Green and red arrows in E indicate the distance between the dorsal midline and the dorsal level of Shh expression in the control (green) and experimental (red) side of an embryo in which a Dkk-soaked bead was implanted (asterisks). Black arrows in E indicate that L-fng encroachment was disrupted in the experimental side of the embryo. Black arrows in F and H indicate the similar distance between the dorsal midline (roof plate) and the dorsal level of Shh expression in the control and experimental side of an embryo in which the PBS-soaked bead was implanted. Black arrows in G indicate the same distance between the dorsal midline (roof plate) and the dorsal level of Shh expression in the control and experimental side of an embryo in which the Dkk-1-soaked bead was implanted in the most caudal position of the alar/roof plate of p2. I, Schematic representation of barrier implantation in HH10 chick embryo. J, Lateral view of the control side of HH23 chick embryos analyzed by double ISH for Shh (blue) and L-fng (red). K, Lateral view of the experimental side of chick embryos. Asterisk in K indicates the region in which the microbarrier was implanted at HH10. Arrows in J and K indicate that the expression of L-fng at both sides of the ZLI was not disrupted in embryos operated with the microbarrier, in which Shh was blocked to progress through the ZLI. Di, Diencephalon; Hy, hypothalamus; Mes, mesencephalon; T, Tel, telencephalon.
Thus, Wnt8b expression labels a central part of the prospective alar plate of the diencephalon at early neurula stage (HH10–HH14), and this expression becomes progressively restricted to the intrathalamic (p3/p2) limit between HH15 and HH17, preconfiguring the ZLI site before Shh expression appears (Figs. 1C, 2K, 3B). At stage HH23, Wnt8b becomes restricted to the ZLI, a part of the p3/p2 roof plate, and a narrow band that connects the basal end of the ZLI with the hypothalamic basal plate expression domain (Figs. 2L, 3D). This dynamic of the expression of Wnt8b attracted our interest to investigate the role of Wnt signals in the mechanisms regulating Shh expression in the ZLI and, subsequently, in the establishment of the ZLI organizer properties.
Signals from the basal plate do not affect Wnt8b expression in the ZLI
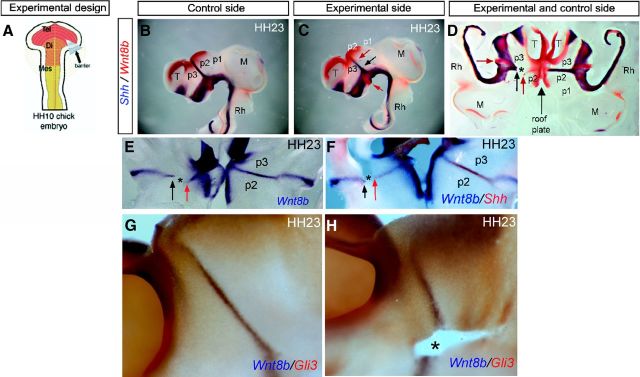
In a first approach, we inserted metal microbarriers in the primordial diencephalon, aiming to mechanically stop any upward cell movement in the prospective ZLI area, as well as any planar inductive interactions between alar and basal neuroepithelial cells, testing in such material the possible modification of Wnt8b expression in the ZLI (Vieira and Martinez, 2006). Microbarriers were inserted between the basal and alar plate at the prospective ZLI location in HH10 chick embryos (Fig. 4A), according to the diencephalic fate map (Garcia-Lopez et al., 2004). Embryos were collected at HH23 and examined to visualize the effect of the barriers on the expression of Shh and Wnt8b using double ISH (Fig. 4B–D).
Figure 4.
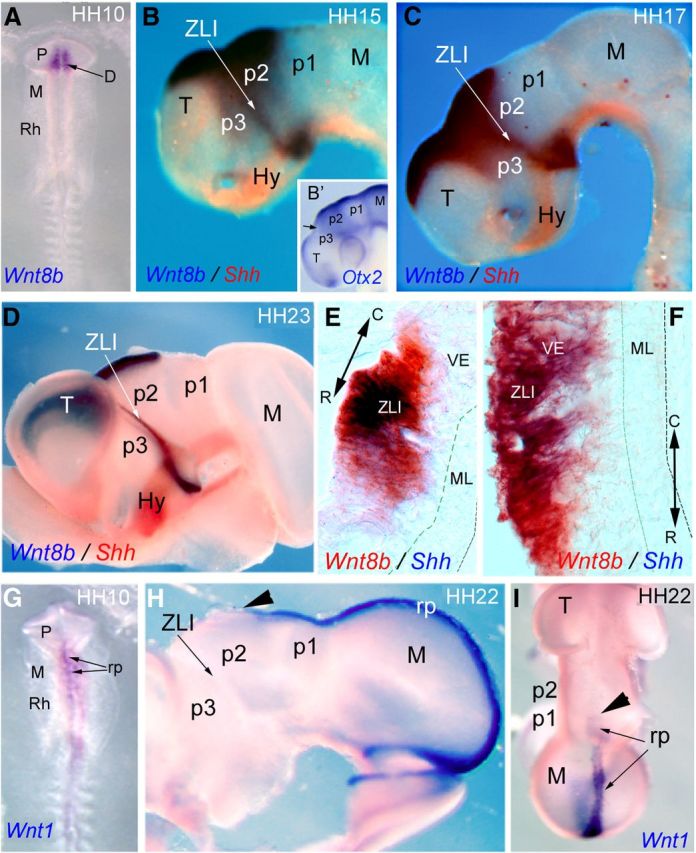
Implantation of microbarriers does not impede Wnt8b expression in the ZLI. A, Schematic representation of barrier implantation in HH10 chick embryo. B, Lateral view of the control side of HH23 chick embryos with double ISH for Shh in blue and Wnt8b in red. C, Lateral view of the experimental side of HH23 chick embryos with double ISH for the indicated genes. Black arrow labels the level at which Shh expression ends in the experimental side; red arrows label Wnt8b expression in the basal and alar plates of the diencephalon. D–F, Dorsal view of the embryos (opened through the ventral midline) analyzed by ISH for Shh in blue and Wnt8b in red (D; the same specimen as that in B, C), Wnt8b in blue (E), and Shh in red (F). C–F, Black arrows indicate the dorsal limit of Shh expression in the experimental ZLI after microbarrier implantation. Red arrows indicate Wnt8b expression in the basal (caudal hypothalamus) and alar diencephalon (p3/p2 limit). G, Lateral view of the control side of HH23 chick embryos with double ISH for Wnt8b in blue and Gli3 in red. H, Lateral view of the experimental side of HH23 chick embryos with double ISH for the indicated genes. Asterisks label microbarrier scars. Di, Diencephalon; M, mesencephalon; T, telencephalon; Rh, rhombencephalon.
Insertion of horizontal barriers was found to block Shh expression in the ZLI neuroepithelium dorsal to the barrier, as observed previously (Vieira and Martinez, 2006). However, we did not observe any effect on Wnt8b expression, either dorsally or ventrally to the barrier (Fig. 4D–H), except some directional anomalies attributable to scar formation in the epithelium around the barrier. Thus, although Shh was impeded from progressing dorsally into the diencephalic alar plate between stages HH17 and HH23, both Wnt8b expression (Fig. 4B–F; n = 6 of 7) and the gap lacking Gli3 expression at the ZLI area were not modified (Fig. 4G,H; n = 4 of 4). Consequently, neither ventral influences, acting through planar induction, nor Shh expression in the incipient ZLI are necessary for Wnt8b expression and associated downregulation of Gli3 at the incipient ZLI.
Inhibition of Wnt8b signaling by implanted Dkk-1 beads
To elucidate whether the observed Wnt signal has incidence on Shh expression in the ZLI, we next decided to block Wnt signaling by implanting Dkk-1-soaked beads in the diencephalic vesicle of HH10 chick embryos. PBS-soaked beads were used as control (Fig. 5A). Dkk-1 protein is a secreted inhibitor of canonical Wnt signaling, which modulates this pathway during embryonic development (Bafico et al., 2001; Mao et al., 2001). We focused our attention on Wnt8b expression because it is the only Wnt gene expressed in the diencephalic area of interest at the experimental stage (HH10). Our results showed that Wnt8b expression was indeed downregulated at the ZLI in the experimental Dkk-treated side in embryos fixed at HH15–HH16 (Fig. 5B,C; n = 3 of 4). Control experiments did not show any effect on Wnt8b expression (Fig. 5D,E; n = 3 of 3). As a control of tissue preservation, we performed double ISH to detect jointly Fgf8 expression, which showed a normal expression pattern in the isthmus, dorsal diencephalon, hypothalamus, and septal telencephalon; this indicates that the experimental procedure did not cause collateral changes in neuroepithelial regionalization. According to these data, Wnt signals are required to maintain Wnt8b expression in the dorsal diencephalon; this suggests a positive autoregulatory (Wnt signaling) dependence mechanism.
Figure 5.
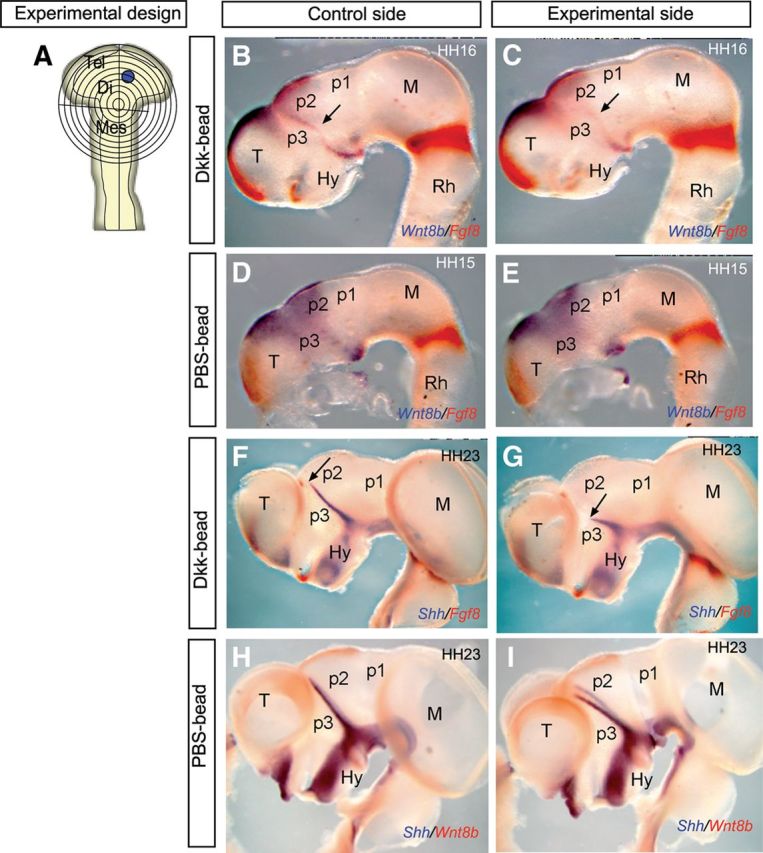
Inhibition of Wnt signaling blocks the dorsal progression of Shh expression in the ZLI. A, Schematic representation of the bead-implanting procedure in which Dkk-1- and PBS-soaked beads were implanted into the prospective ZLI of chick embryos at HH10 (anterior pole of Wnt8b expression domain). B, D, F, H, Lateral views of the control sides of the chick embryos analyzed by double ISH for the indicated genes in each picture. C, E, G, I, Lateral view of the experimental side of chick embryos analyzed by double ISH for the indicated genes in each picture. B, C, Embryos implanted with Dkk-1-soaked beads and analyzed for Wnt8b and Fgf8 expression 24 h after bead implantation. Arrows indicate Wnt8b expression in the ZLI (B) and its absence in the experimental side (C). D, E, Embryos implanted with PBS-soaked beads and analyzed for Wnt8b and Fgf8 expression 24 h after bead implantation, showing similar expression of Wnt8b in the diencephalon. F, G, Embryos implanted with Dkk-1-soaked beads and analyzed for Shh and Fgf8 expression 4 d after bead implantation. Arrow in F and G indicates the most dorsal level of Shh expression in the ZLI. Note the dorsal reduction of Shh expression in the experimental ZLI. H, I, Embryos implanted with PBS-soaked beads and analyzed for Shh and Wnt8b expression 4 d after bead implantation, showing normal expression of Shh in control and experimental sides. Di, Diencephalon; Hy, hypothalamus; M, Mes mesencephalon; Rh, rhombencephalon; T, Tel telencephalon.
Shh induction in the ZLI requires Wnt8b expression
Several studies have suggested that dorsal diencephalic tissue counteracts ZLI formation (Zeltser, 2005; Guinazu et al., 2007). The molecules secreted in the dorsal diencephalon include various members of the Bmp and Wnt families, as well as retinoic acid (Zeltser, 2005). These molecules hypothetically would inhibit Shh expression in this region. However, electroporation experiments using activators of the Wnt pathway do not inhibit Shh expression in the ZLI (Guinazu et al., 2007), suggesting that Wnt does not mediate the described inhibitory effect of dorsal diencephalic tissue. This is in agreement with our observations in which coexpression of Wnt8b and Shh was observed in ZLI epithelial cells (Fig. 3D–F). Furthermore, the expression of Wnt8b in the ZLI before Shh is activated also suggests that Wnt signals may rather play a positive role in this process. Therefore, we investigated whether Wnt signals generate a permissive territory for the ventrodorsal activation of Shh in the ZLI. Dkk-1 bead implantation experiments into the prospective ZLI at HH10 were analyzed after 4 d of postoperative incubation (Fig. 5F,G). Dkk-1 beads implanted in the prospective ZLI locus limited the dorsal progression of Shh expression at the ZLI (Fig. 5F,G; n = 4 of 5), whereas no effect was detected by inserting control (PBS-soaked) beads (Fig. 5H,I; n = 3 of 3). Therefore, Wnt signaling, probably represented by Wnt8b expression, is required in the diencephalon for Shh activation in ZLI neuroepithelial cells. Insertion of Dkk-1 beads in different areas of prospective p2 and p1, to exclude additional Wnt inductive effects from more caudal regions, in which Wnt1 and Wnt3a are later expressed (Fig. 2), did not modify Shh expression at the ZLI (see Fig. 8G,H; n = 5 of 5).
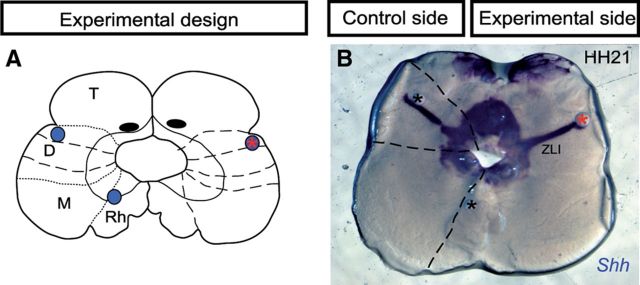
Next we studied whether the maintenance of Shh expression in the ZLI also requires Wnt signal. To assess this aspect, we prepared organotypic half-brain cultures from dissected chick neural tube at HH21 (Echevarría et al., 2001). Dkk-1 beads were inserted into the dorsal part of the ZLI, and the specimens were cultured for 24 h (Fig. 6). We observed that neither Dkk-1 nor PBS beads produced downregulation of Shh in the ZLI (Fig. 6B; n = 8 of 8). Thus, Wnt activity, despite being required for Shh activation in the ZLI epithelium, is not required for the maintenance of its expression.
Figure 6.
Wnt signaling is not required for the maintenance of Shh expression in the ZLI. A, Schematic representation of the neural tube explants in which Dkk-1 or PBS-soaked beads were inserted at the left or right side, respectively. B, Shh expression in HH21 cultured explants 24 h after culture. PBS-soaked beads (black asterisk) or Dkk-1-soaked beads (red asterisk) were inserted into the dorsal region of the ZLI of chick embryos at stage HH21. No modification of Shh expression was observed after blocking Wnt signaling in the experimental side. D, Diencephalon; M, mesencephalon; T, telencephalon; Rh, rhombencephalon.
Wnt signal controls Gli3 expression by restricting spatially Shh/Gli activity
In agreement with Hashimoto-Torii et al. (2003), we observed that Gli3 was differentially expressed in the developing alar diencephalon before Shh becomes expressed at the ZLI (Fig. 1A). In the HH15–HH16 chick diencephalon, a transverse alar stripe that is devoid of Gli3 expression appears associated with the prospective locus of the ZLI expressing Wnt8b (Fig. 1A,D,G). We explored the possibility that Wnt signals may be downregulating Gli3 expression at this locus, as is known to occur in the spinal cord (Alvarez-Medina et al., 2008). Therefore, we analyzed Gli3 expression in the prospective ZLI after Dkk-1-bead implantation (Fig. 7A–C). This resulted in abnormal Gli3 expression across the presumptive ZLI neuropithelium (Fig. 7B,C; n = 7 of 8). This result suggests that Wnt signaling is required for inhibition of Gli3 expression in the prospective ZLI. The downstream repression of Gli3 in the ZLI suggests a role of Wnt8b in establishing a permissive territory, which subsequently may allow the ventrodorsal progression of Shh expression. To test whether ectopic expression of Wnt8b generally causes Gli3 repression and Shh induction, we grafted Wnt8b-expressing quail diencephalic neuroepithelium into the chick mesencephalon at HH10 (Fig. 7D). We describe here only grafts made into rostral mesencephalic areas because we found that Wnt8b expression in the donor tissue was not maintained if placed within the caudal diencephalon (data not shown). Normally, the mesencephalic alar plate homogenously expresses Gli3 from stage HH15 onward (Fig. 1A,D,G). The operated embryos were allowed to develop for 2 d after the graft. We examined the chimeric embryos by ISH for Gli3, Shh, or Wnt8b and by immunohistochemistry for QCPN (Fig. 7E–O). Two effects were observed: Gli3 expression was repressed around the grafts, which continued expressing Wnt8b (Fig. 7E–K), and Shh expression was induced in the neighboring host tissue (QCPN-negative epithelium) (Fig. 7H–K; n = 5 of 7). This result indicates that the transplanted tissue expressing Wnt8b (but not Shh) was capable of regulating in the host mesencephalon the expression of Gli3 and Shh: repressing Gli3 and activating Shh.
Figure 7.
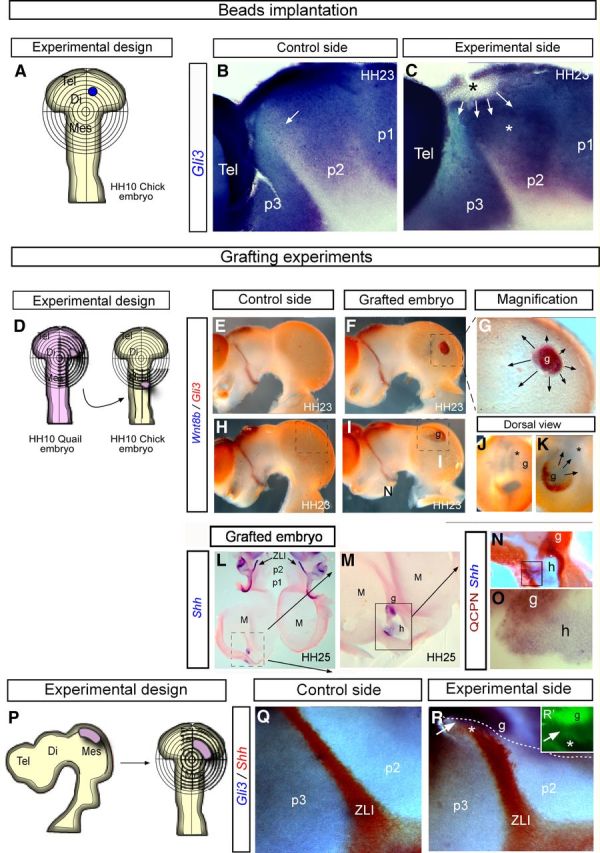
Wnt8b downregulates Gli3 expression in the alar plate. A–C, Inhibition of Wnt8b maintains Gli3 expression in the prospective ZLI. A, Schematic representation of the bead-implanting procedure in which Dkk-1- and PBS-soaked beads were implanted into the ZLI of chick embryos at HH10. B, C, Lateral views of the control side (B) and experimental side (C) of the chick embryos neural tube analyzed by ISH for Gli3 in blue. Arrows in B localize the ZLI, in which Gli3 is not expressed. In C, Gli3 is overexpressed in the diencephalic alar plate, making it impossible to distinguish the p3/p2 boundary in the most dorsal domain. D–O, Grafting experiments. D, Schematic representation of the grafting procedure in which Wnt8b-expressing neuroepithelium was transplanted from HH10 quail embryos into the alar plate of the mesencephalic vesicle of HH10 embryos. E, H, Lateral view of the control side of the neural tube at HH23 showing double ISH for Wnt8b (blue) and Gli3 (red). F, I, Lateral views of the experimental side of the chimeric neural tube at HH23, analyzed by double ISH for Wnt8b (blue) and Gli3 (red). G, Higher-magnification view of the square represented in F. J, K, Dorsal view of control (J) and grafted (K) embryos, showing the expression of Gli3 in the mesencephalon and its repression around the Wnt8b graft. L–O, The host in which Gli3 was downregulated activated Shh expression. L, Flat mount of the chimeric neural tube, opened through the ventral midline and analyzed for Shh by ISH. M, N, Higher-magnification view of the square represented in L. M, Shows Shh ectopic activation in the mesencephalic alar plate. N, O, Higher-magnification views of the same embryo shown in L, analyzed by ISH for Shh in the QCPN-negative (host) territory. Quail cells (maroon) do not expressed Shh (blue). P, Schematic representation of the grafting procedure in which Gli3-expressing neuroepithelium was transplanted from HH23 quail embryos into the alar plate of the diencephalic vesicle of HH10 embryos. Q–R, Lateral views of the control side (Q) and experimental side (R) of the chick embryos neural tube analyzed by double ISH for Gli3 in blue and Shh in red; R′, QCPN+ cells were detected by immunofluorescence. White arrows in R and R′ localize host cells expressing Gli3. Di, Diencephalon; g, graft tissue; h, host tissue; M, Mes, mesencephalon; Tel, telencephalon.
To explore whether Gli3-expressing neuroepithelium was sufficient to stop upward Shh induction in the primordial ZLI, we grafted alar mesencephalon of HH23 quail embryos into the dorsal p2 region of chick embryos at HH10 stage (Fig. 7P). After 3 d of survival, we observed continued expression of Gli3 and a failure of Shh induction in the graft-derived area of chimeric embryos (Fig. 7R; 5 of 5). Moreover, a narrow epithelial band of the host diencephalic epithelium was induced to express Gli3 at the graft/host boundary; these host cells also did not activate Shh expression (Fig. 7R,R′, white arrow; n = 3 of 5). Undoubtedly, the replacement of the Wnt8b-expressing intrathalamic domain by Wnt8b-negative donor mesencephalic epithelium eliminated required Wnt signals and, consequently, Gli3 expression is maintained at the grafted mesencephalon and neighboring host epithelium (Fig. 7P–R). The possibility of an experimental artifact is discarded, because inductive planar interactions between quail and chick neuroepithelial cells have been corroborated extensively in the diencephalon (Vieira and Martinez, 2006), and chimeras carrying diencephalic homotopic grafts develop normally (Garcia-Lopez et al., 2004).
Wnt8b defines L-fng expression domain
We decided to further analyze whether other molecules that control Shh progression in the ZLI may be also under the control of Wnt signaling. L-fng is expressed in the diencephalon flanking the ZLI once this is formed and is normally dynamically complementary to Wnt8b expression in the diencephalon, expanding as the latter becomes restricted at the ZLI, thus progressively encroaching on the latter (Garcia-Lopez et al., 2004). The molecular and cellular mechanisms regulating this dynamic L-fng expression pattern are still unknown. Moreover, previous studies showed that L-fng can repress Shh expression at the ZLI (Zeltser et al., 2001). To determine whether Wnt signals influence L-fng expression, we inserted Dkk-1- and PBS-soaked beads into the prospective ZLI of chick embryos at HH10 and analyzed L-fng and Shh expression 4 d after bead implantation (Fig. 8). We observed that the encroachment of the L-fng-expressing domain on the dorsal part of the ZLI was impeded in the experimental side, associated with a lack of Shh induction at the dorsal tip of the ZLI (Fig. 8A–F; n = 10 of 13). When a Dkk-1 bead was implanted in the caudal diencephalon (counteracting other potential Wnt signals), the encroachment of L-fng around Shh expression in the ZLI was not abolished (Fig. 8G,H; n = 4 of 4). Conversely, when only Shh expression at the ZLI was abolished by insertion of a horizontal barrier, a normal L-fng expression pattern was observed, suggesting that the encroaching L-fng expression is regulated by the signal present at the p2/p3 limit and not by secondary Shh signal (Fig. 8I–K; n = 4 of 5).
The p3/p2 boundary itself is regulated by Wnt signaling
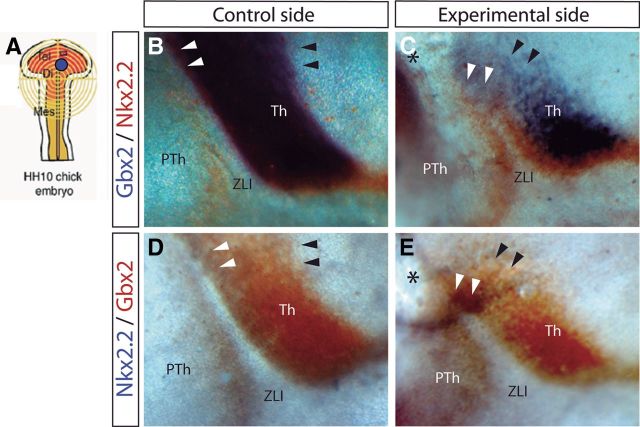
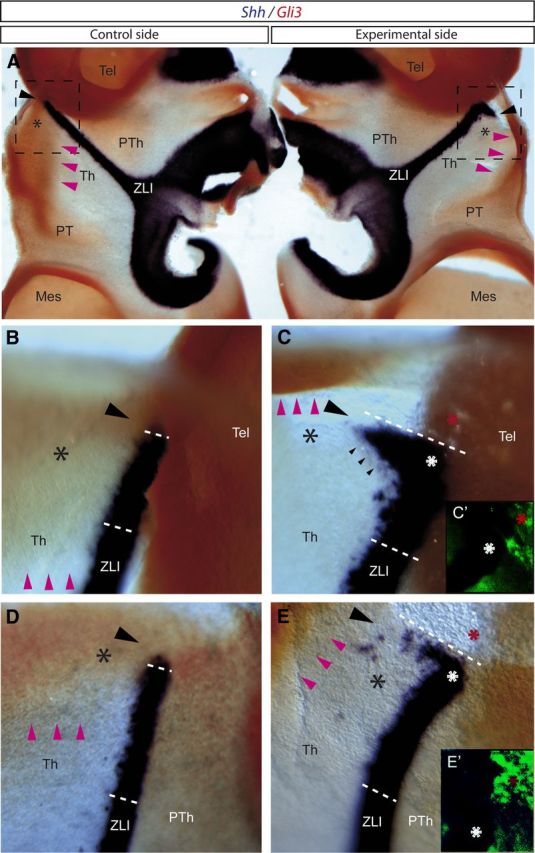
It is known that Shh acts in the ZLI as a signaling molecule that regulates the expression of developmental genes that will specify compartmentalization of neighboring diencephalic progenitor areas (Echevarría et al., 2003; Vieira et al., 2005), such as domains expressing Gbx2 or Nkx2.2. Gbx2 is a homeobox gene known to be expressed in thalamic mantle layer cells, and some studies have demonstrated a positive regulation of Gbx2 by Shh (Hashimoto-Torii et al., 2003; Kiecker and Lumsden, 2004; Vieira and Martinez, 2006). Moreover, Nkx2.2 is a homeodomain transcription factor (TF) expressed in the diencephalon separating the basal and alar plates (Shimamura et al., 1995) and also flanking the ulterior expression of Shh in the ZLI (Martínez-de-la-Torre et al., 2002). As expected (Vieira et al., 2005), when we implanted Dkk-1-soaked beads in the dorsal diencephalon and observed a truncation of ZLI Shh induction (Figs. 5C, 8E), there also appeared a dorsal zone with corresponding reduction of Gbx2 and Nkx2.2 expression (Fig. 9, black arrowheads; n = 5 of 8).
Figure 9.
Inhibition of Wnt signal results in downregulation and ectopic expression of Gbx2 and Nkx2.2. A, Schematic representation of the neural tube explants in which Dkk-1 was inserted. B–E, Lateral view of the control side (B, D) and experimental side (C, E) of chick embryos neural tube analyzed by ISH for Gbx2 in blue and Nkx2.2 in red (B, C) and for Nkx2.2 in blue and Gbx2 in red (D, E). Black arrowheads localize the caudal limit of Gbx2 expression (see its reduction in C and E), whereas white arrowheads localize the region in the dorsal ZLI in which Gbx2 and Nkx2.2 were ectopically expressed. Black asterisks indicate the place in which Dkk-1-soaked beads were implanted. Th, Thalamus; PTh, prethalamus.
Surprisingly, the experimental side of the embryos receiving Dkk-1 beads often showed an abnormal expression of Nkx2.2 and Gbx2 covering the dorsal tip of the stunted ZLI (Fig. 9, white arrowheads; n = 5 of 8), forming a bridge between p2 and p3; clearly the p3/p2 boundary in not established in the absence of Wnt signal.
An ectopic source of Wnt signal induces molecular reorganization in the host tissue
Our experiments strongly suggested that a Wnt signal is required as first permissive step for the subsequent activation of Shh expression in the ZLI; this effect is thought to occur via locally restricted negative regulation of Gli3 expression at the center of the early Wnt8b expression domain. To confirm this hypothesis, we implanted Wnt1-expressing cells in the dorsal diencephalon of chick embryos at HH10 (we do not have cells expressing Wnt8b; it was hoped that other Wnts signals can duplicate the postulated Wnt8b function). After 3 d of incubation, we explored the effect of supposedly physiological levels of ectopic Wnt1 signal on Gli3 and Shh expression. In the dorsal diencephalon of the experimental side, we observed a reduction of the domain of Gli3 expression in the dorsal thalamus (Fig. 10, pink arrowheads; n = 5 of 7), together with an ectopic expression of Shh in this area (Fig. 10, black arrowheads; n = 5 of 7).
Figure 10.
Transplantation of Wnt1-expressing cells results in repression of Gli3 expression and induction of Shh expression in the dorsal region of the thalamus. A–E, Embryos analyzed by ISH for Shh in blue and Gli3 in red. A, Dorsal view of embryos (opened through the midline) analyzed by ISH for Shh and Gli3. B, C, Magnification of the squares represented in A. B, D, Control side of embryos transplanted with Wnt1-expressing cells. C, E, Experimental side of embryos transplanted with Wnt1-expressing cells. C′, E′, Immunofluorescence for QCPN. Black asterisks mark the most dorsal region of the thalamus, in which Gli3 is normally expressed (see control side in A, B, D). Pink arrowheads label the ventral limit of Gli3 expression in the thalamus. Increasing negative gap of Gli3 expression is observed in the experimental side of the embryos (see pink arrowheads in C and E compared with B and D, respectively). Black arrowheads indicate the region in which ectopic Shh expression is observed near to the place where Wnt1-expressing cells were transplanted (red asterisks in C, C′, E, E′). White asterisks (C, E) indicate the most dorsal domain of the ZLI. Mes, Mesencephalon; Tel, telencephalon; Th, thalamus; PT, pretectum; PTh, prethalamus.
Thus, Wnt signals operating in the ZLI seem to regulate both the early downregulation of Gli3, which allows ventrodorsal homeiotic Shh induction from underlying basal Shh-positive domain, and parallel encroachment of L-fng on the forming ZLI (Fig. 11). This Wnt activity is held to be mediated normally by Wnt8b, because it is the main Wnt gene expressed at the ZLI before Shh is expressed in that area.
Figure 11.
Schematic representation illustrating regulatory interactions between morphogenes and TFs acting in the ZLI. A, Drawing of chick embryo prosencephalon (stage HH23) showing the relationship of the ZLI with diencephalic prosomeres. Wnt8b and Shh expression have been represented by color codes. B, Gene expression pattern of different genes (names and color codes) in the diencephalic neuroepithelium. C, Gene expression pattern in the diencephalon after Shh abolition in the ZLI by metal microbarrier insertion, showing that the limit between p2/p3 was not disrupted: Wnt8b was normally expressed in the ZLI, and L-fng showed normal expression patterns. D, Gene expression pattern in the diencephalon after Wnt8b inhibition in the dorsal ZLI by Dkk-1-soaked bead implantation. The negative gap of Gli3 expression in the ZLI was abolished. In the contrast, encroachment of L-fng expression was impeded. These two effects, mediated by Wnt8b signal, impede Shh progression in the ZLI from the basal plate. E, Mechanicistic model interpreting our results: Wnt8b morphogenetic gradient from the ZLI (blue gradient) controls the expression of diencephalic genes and is fundamental for the correct specification of the Shh expression in the ZLI in chick embryos. The arrows show interactions (activation or repression) between diencephalic signals: green arrows represent L-fng induction, and green arrows with lines represent Gli3 inhibition by Wnt8b signaling. Then, the negative gap of Gli3 expression and an encroachment of L-fng expression in the ZLI generated a permissive territory (free from repressive activity of Gli3, red arrows with lines) for Shh expression in this region, which progresses by planar auto-inductive mechanisms from the basal plate (red arrows). ap, Alar plate; BP, basal plate.
Discussion
The ZLI is an important diencephalic organizer (for review, see Scholpp and Lumsden, 2010; Martinez-Ferre and Martinez, 2012). Apart from the gradiental distribution of Shh spreading from the ZLI and the underlying basal plate, relevant positional information is provided also by other signaling molecules, such as Fgfs (Kataoka and Shimogori, 2008; Martinez-Ferre and Martinez, 2009), Bmp (Furuta et al., 1997; Lim et al., 2005), and Wnts (Braun et al., 2003; Zhou et al., 2004; Bluske et al., 2009). These jointly modulate the regional expression of various TFs, whose downstream effects regulate proliferation (Juraver-Geslin et al., 2011) and cell differentiation in the prethalamus and thalamus. The singularity of the ZLI, as a transversal spike apparently arising from the longitudinal basal plate, has long attracted attention.
Molecular mechanisms positioning the ZLI
Analogously to the mechanism regulating the position and fate specification of the isthmic organizer at the interface of Otx2- and Gbx2-expressing domains (midbrain/hindbrain), the diencephalic ZLI was proposed to result from the interaction between prechordal (Six3- and Fez-positive) and epichordal (Irx3-positive) regions of the forebrain [Kobayashi et al., 2002; Vieira et al., 2005, Guinazu et al., 2007; note that a new definition of the epichordal floor plate, bringing its rostral end to the mammillary midline (Puelles et al., 2012), implies that the so-called “prechordal territory” now represents the rostral forebrain]. Nevertheless, Six3 is not required for the ZLI formation in mice (Lagutin et al., 2003; Lavado et al., 2008). Thus, ZLI develops at the interface between prethalamic and thalamic prosencephalic regions expressing heterogeneous TFs that may contribute to ZLI dimensioning in width and confer differential competence to respond to any organizer signals (Vieira and Martinez, 2006).
In the chick neural tube, Wnt8b expression represents the earliest distinct marker for the diencephalon. Between HH10 and HH15, its transverse wedge-shaped expression domain collapses into a narrow strip along the ZLI, which contracts from ventral to dorsal. This collapse may be the result of repressive effects occurring within the flanking domains, after activation of TFs such as Fez, Lrrn1, Otx2, and Irx3, or attributable to mutual inhibition with Gli3 (the latter is repressed at the center of the Wnt8b domain, whereas Wnt8b is downregulated at the periphery). Later on, between HH17 and HH25, the ZLI begins to express Shh (Fig. 3). Therefore, the early and dynamic expression of Wnt8b in the diencephalic wall may represent the initial condition for p3/p2 boundary positioning and ZLI formation.
Kiecker and Lumsden (2004) proposed that the dynamic expression pattern of L-fng in the diencephalon (with progressive encroachment on the forming ZLI) plays a key role in the induction of Shh in the ZLI. Because L-fng encroachment is not attributable to either cellular movements in the epithelium (Garcia-Lopez et al., 2004) or cell death (Zeltser et al., 2001), it must be the consequence of progressive rostralward induction of L-fng into thalamic areas, starting from an initial pretectal (p1) diencephalic alar territory (Zeltser et al., 2001; Ferran et al., 2007). L-fng expression in the prethalamus does not change, so that all the encroachment occurs caudal to the ZLI. L-fng probably expands into the thalamus region in the wake of the collapsing Wnt8b domain at the ZLI, because Wnt8b is needed for L-fng expression, as we showed. This interpretation suggests that L-fng plays a passive role relative to the formation of the ZLI, even if it later modulates its Shh expression and boundary properties. Although the contracting L-fng-negative neuroepithelium might be seen as a domain permissive for Shh expression, this is contradicted by the lack of early induction of Shh within it, probably because of the widespread alar expression of Gli3, a result of early ventrodorsal patterning. Secondary repression of Gli3 along the center of the transverse Wnt8b expression domain, first visible at HH15, seems instead crucial to allow the Shh-positive basal domain to selectively induce Shh homeotically within the Gli3-devoid stripe.
Interestingly, whereas in the chicken ZLI planar induction out of the Shh-expressing basal domain is required for the dorsalward progression of Shh expression (Vieira and Martinez 2006; present results), a dorsal Wnt signal (probably accompanied by Gli3 repression) seems sufficient to activate Shh at the ZLI locus in the one-eye-pinhead zebrafish mutant, which lacks a basal plate (Scholpp et al., 2006). Species differences in the function of a specific enhancer regulating Shh expression at the ZLI (Jeong et al., 2011) might be the cause of this discrepancy.
Other signals contribute to define the width of the ZLI compartment
In this work, we propose that the ZLI compartment expressing Shh is positioned and crucially allowed to emerge by Wnt-mediated signals leading to localized repression of Gli3 rather than by dynamic expression of L-fng (Zeltser et al., 2001). An additional role may be played by the early expression of Lrrn1 just rostral to the ZLI; the latter precedes Shh induction and intriguingly is found likewise at the isthmic organizer (García-Calero et al., 2006; Andreae et al., 2007; Tossell et al., 2011).
Shh is not expressed throughout the ZLI in the LRP6 mutant mice (Zhou et al., 2004; LRP6 is a Wnt coreceptor) or after blockage of Wnt signaling (present results; Fig. 5F–I), and normal Wnt signaling at early neural tube stages seems to be required for the establishment of an Shh/Gli3-negative primordial ZLI region at the alar p3/p2 boundary. Guinazu et al. (2007) performed electroporation experiments at stage HH16 using constructs that activated or blocked the Wnt/β-catenin pathway. They reported that thalamic gene expression is not directly or indirectly regulated by Wnt proteins. This result is not contradictory with our conclusion above, because the ZLI probably was already established before Wnt signaling was modified in their experiments. Inhibition of the Wnt pathway once Shh is already expressed in the ZLI does not have any effect on its maintenance (Fig. 6).
Other molecular determinants seem to collaborate with Wnt8b in the ZLI specification process. It was demonstrated in zebrafish embryos that the prethalamus plays a role in ZLI formation by means of frizzled-receptor-mediated blockage of Wnt signaling (Peng and Westerfield, 2006; Tendeng and Houart, 2006; Jeong et al., 2007). This mechanism would contribute to the shrinkage of Wnt8b expression, thus dimensioning the width of the ZLI, together with caudal signals such as Irx3 acting at the thalamic side (p2). The latter effect was evidenced in our grafts of prospective ZLI tissue into caudal diencephalon, which lost their Wnt8b expression. Moreover, in zebrafish, Otx1l and Otx2 expression in the diencephalon determines a competent domain in which a ZLI is induced (Scholpp et al., 2007). However, the homogeneous expression of Otx2 throughout the chick diencephalon at stages HH15–HH17 does not suggest a relevant positional role of this gene in specifying the neuroepithelial corridor in which Shh will be induced, although it may be needed to underpin the interactions between Wnt8b and the other protagonists, including Gli3. As the ZLI matures, after Shh expression begins, Otx2 expression is upregulated at its neuroepithelium (Crossley et al., 2001).
Wnt/β-catenin, L-fng, and Shh/Gli signaling pathway during the establishment of Shh expression in the ZLI
Previous studies have demonstrated that Gli3 is required for Wnt gene expression at various brain locations (Grove et al., 1998; Mullor et al., 2001; Ulloa et al., 2007). Moreover, Alvarez-Medina et al. (2008) showed that Wnt activity is required for Gli3 expression. In contrast to this positive mutual regulation, at the chicken ZLI, the Gli3 and Wnt8b genes are complementarily expressed. When Wnt canonical signaling does not oppose the expression of Gli3 at the prospective ZLI, Shh is not activated in the alar diencephalon (Fig. 5). Thus, because the expression of Gli3 (the main transcriptional repressor of the Shh/Gli pathway) in the ZLI inhibits Shh induction, the observed early downregulation of Gli3 by Wnt8b signal is a key process in the diencephalon for establishment of the ZLI organizer and secondary induction of Shh. Although Wnt8b signal seems necessary for positionally restricted downregulation of Gli3 at the primordial ZLI, it is not sufficient, as is indicated by the fact that the whole domain that expresses Wnt8b does not lose Gli3 expression. A combination of factors acting at the early interface between prethalamic (Six3+, Lrrn1+, Fez+) and thalamic (Irx3+) prosencephalic neuroepithelium apparently contributes to the dimensioning of the Gli3-negative primordial ZLI.
We conclude that Wnt8b expression in the alar diencephalon underlies jointly with other molecular interfaces (Fez, Lrrn1, Otx2, and Irx3) the p3/p2 boundary formation and subsequently underpins at its center the required molecular events that result in a permissive Gli3-negative transverse spike (the primordial ZLI), in which basal plate inductive signals (Shh) generate the definitive ZLI (Fig. 11). The results described here demonstrate a regulation of L-fng and Gli3 expressions by Wnt signaling in the alar diencephalon. L-fng modulates Notch signaling at the boundary cells (Tossell et al., 2011), thus contributing to specification of the ZLI borders (ZLI width; Larsen et al., 2001; Zeltser et al., 2001). In parallel, by repressing Gli3 expression, Wnt signaling crucially generates a permissive territory at the p3/p2 boundary, the primordial ZLI, in which Shh results activated ventrodorsally by planar inductive mechanisms (Fig. 11).
Footnotes
This work was supported by EUCOMMTOOLS Contract 261492, Spanish Ministry of Science and Innovation Grant BFU-2008-00588, Ministry of Education and Science–Universitary Professor Formation Grant AP2009-3644, Consolider Grant CSD2007-00023, Institute of Health Carlos III, Spanish Cell Therapy Network and Research Center of Mental Health, General Council of Valencia (Prometeo 2009/028 and 11/2011/042), and the Alicia Koplowitz Foundation. We thank M. Ródenas for technical assistance.
References
- Abbasi AA, Paparidis Z, Malik S, Bangs F, Schmidt A, Koch S, Lopez-Rios J, Grzeschik KH. Human intronic enhancers control distinct sub-domains of Gli3 expression during mouse CNS and limb development. BMC Dev Biol. 2010;10:44. doi: 10.1186/1471-213X-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Medina R, Cayuso J, Okubo T, Takada S, Martí E. Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development. 2008;135:237–247. doi: 10.1242/dev.012054. [DOI] [PubMed] [Google Scholar]
- Andreae LC, Peukert D, Lumsden A, Gilthorpe JD. Analysis of Lrrn1 expression and its relationship to neuromeric boundaries during chick neural development. Neural Dev. 2007;31:2–22. doi: 10.1186/1749-8104-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman M, Machon O, Mygland L, van der Bout CJ, Zhong W, Taketo MM, Krauss S. Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Dev Biol. 2005;279:155–168. doi: 10.1016/j.ydbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Bluske KK, Kawakami Y, Koyano-Nakagawa N, Nakagawa Y. Differential activity of Wnt/beta-catenin signaling in the embryonic mouse thalamus. Dev Dyn. 2009;238:3297–3309. doi: 10.1002/dvdy.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun MM, Etheridge A, Bernard A, Robertson CP, Roelink H. Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development. 2003;130:5579–5587. doi: 10.1242/dev.00685. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Martin GR. Midbrain development induced by FGF8 in the chick embryo. Nature. 1996;7:66–68. doi: 10.1038/380066a0. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Ohkubo Y, Rubenstein JL. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108:183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- Echevarría D, Vieira C, Martínez S. Mammalian neural tube grafting experiments: an in vitro system for mouse experimental embryology. Int J Dev Biol. 2001;45:895–902. [PubMed] [Google Scholar]
- Echevarría D, Vieira C, Gimeno L, Martínez S. Neuroepithelial secondary organizers and cell fate specification in the developing brain. Brain Res Rev. 2003;43:179–191. doi: 10.1016/j.brainresrev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- Ferran JL, Sánchez-Arrones L, Sandoval JE, Puelles L. A model of early molecular regionalization in the chicken embryonic pretectum. J Comp Neurol. 2007;505:379–403. doi: 10.1002/cne.21493. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- García-Calero E, Garda AL, Marín F, Puelles L. Expression of Lrrn1 marks the prospective site of the zona limitans thalami in the early embryonic chicken diencephalon. Gene Exp Patterns. 2006;6:879–885. doi: 10.1016/j.modgep.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Garcia-Lopez R, Vieira C, Echevarria D, Martinez S. Fate map of the diencephalon and the zona limitans at the 10-somites stage in chick embryos. Dev Biol. 2004;268:514–530. doi: 10.1016/j.ydbio.2003.12.038. [DOI] [PubMed] [Google Scholar]
- Garda AL, Puelles L, Rubenstein JL, Medina L. Expression patterns of Wnt8b and Wnt7b in the chicken embryonic brain suggest a correlation with forebrain patterning centers and morphogenesis. Neuroscience. 2002;113:689–698. doi: 10.1016/s0306-4522(02)00171-9. [DOI] [PubMed] [Google Scholar]
- Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- Guinazu MF, Chambers D, Lumsden A, Kiecker C. Tissue interactions in the developing chick diencephalon. Neural Dev. 2007;2:25. doi: 10.1186/1749-8104-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Torii K, Motoyama J, Hui CC, Kuroiwa A, Nakafuku M, Shimamura K. Differential activities of Sonic hedgehog mediated by Gli transcription factors define distinct neuronal subtypes in the dorsal thalamus. Mech Dev. 2003;120:1097–1111. doi: 10.1016/j.mod.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Hollyday M, McMahon JA, McMahon AP. Wnt expression patterns in chick embryos nervous system. Mech Dev. 1995;52:9–25. doi: 10.1016/0925-4773(95)00385-e. [DOI] [PubMed] [Google Scholar]
- Jeong JY, Einhorn Z, Mathur P, Chen L, Lee S, Kawakami K, Guo S. Patterning the zebrafish diencephalon by the conserved zinc-finger protein Fezl. Development. 2007;134:127–136. doi: 10.1242/dev.02705. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Leskow FC, EI-Jaick K, Roessler E, Muenke M, Yocum A, Dubourg C, Li X, Geng X, Oliver G, Epstein DJ. Regulation of a remote Shh forebrain enhancer by the Six3 homeoprotein. Nat Genet. 2008;40:1348–1353. doi: 10.1038/ng.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Juraver-Geslin HA, Ausseil JJ, Wassef M, Durand BC. Barhl2 limits growth of the diencephalic primordium through Caspase3 inhibition of beta-catenin activation. Proc Natl Acad Sci U S A. 2011;108:2288–2293. doi: 10.1073/pnas.1014017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka A, Shimogori T. Fgf8 controls regional identity in the developing thalamus. Dev. 2008;135:2873–2881. doi: 10.1242/dev.021618. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Lumsden A. Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat Neurosci. 2004;7:1242–1249. doi: 10.1038/nn1338. [DOI] [PubMed] [Google Scholar]
- Kobayashi D, Kobayashi M, Matsumoto K, Ogura T, Nakafuku M, Shimamura K. Early subdivisions in the neural plate define distinct competence for inductive signals. Development. 2002;129:83–93. doi: 10.1242/dev.129.1.83. [DOI] [PubMed] [Google Scholar]
- Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, Puelles L, Russell HR, McKinnon PJ, Solnica-Krezel L, Oliver G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17:368–379. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CW, Zeltser LM, Lumsden A. Boundary formation and compartition in the avian diencephalon. J Neurosci. 2001;21:4699–4711. doi: 10.1523/JNEUROSCI.21-13-04699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, Lagutin OV, Oliver G. Six3 inactivation causes progressive caudalization and aberrant patterning of the mammalian diencephalon. Development. 2008;135:441–450. doi: 10.1242/dev.010082. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1999;22:261–294. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- Lim Y, Cho G, Minarcik J, Golden J. Altered BMP signaling disrupts chick diencephalic development. Mech Dev. 2005;122:603–620. doi: 10.1016/j.mod.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Martínez S, Puelles L. Neurogenetic compartments of the mouse diencephalon and some characteristic gene expression patterns. Results Probl Cell Differ. 2000;30:91–106. doi: 10.1007/978-3-540-48002-0_4. [DOI] [PubMed] [Google Scholar]
- Martinez S, Puelles E, Puelles L, Echevarria D. Molecular regionalization of the developing neural tube. In: Watson C, Paxinos G, Puelles L, editors. The mouse nervous system. San Diego: Academic; 2012. pp. 2–15. [Google Scholar]
- Martinez-de-la-Torre M, Garda AL, Puelles E, Puelles L. Gbx2 expression in the late embryonic chick dorsal thalamus. Brain Res Bull. 2002;57:435–438. doi: 10.1016/s0361-9230(01)00721-3. [DOI] [PubMed] [Google Scholar]
- Martinez-Ferre A, Martinez S. The development of the thalamic motor learning area is regulated by Fgf8 expression. J Neurosci. 2009;29:13389–13400. doi: 10.1523/JNEUROSCI.2625-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Ferre A, Martinez S. Molecular regionalization of the diencephalon. Front Neurosci. 2012;6:73. doi: 10.3389/fnins.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullor JL, Dahmane N, Sun T, Ruiz i Altaba A. Wnt signals are targets and mediators of Gli function. Curr Biol. 2001;11:769–773. doi: 10.1016/s0960-9822(01)00229-9. [DOI] [PubMed] [Google Scholar]
- Peng G, Westerfield M. Lhx5 promotes forebrain development and activates transcription of secreted Wnt antagonists. Development. 2006;133:3191–3200. doi: 10.1242/dev.02485. [DOI] [PubMed] [Google Scholar]
- Persson M, Stamataki D, te Welscher P, Andersson E, Böse J, Rüther U, Ericson J, Briscoe J. Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev. 2002;16:2865–2878. doi: 10.1101/gad.243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles L, Amat JA, Martinez-de-la-Torre M. Segment-related, mosaic neurogenetic pattern in the forebrain and mesencephalon of early chick embryos. I. Topography of AChE-positive neuroblasts up to stage HH18. J Comp Neurol. 1987;266:247–268. doi: 10.1002/cne.902660210. [DOI] [PubMed] [Google Scholar]
- Puelles L, Javier Milán F, Martínez-de-la-Torre M. A segmental map of architectonic subdivisions in the diencephalon of the frog Rana perezi: acetylcholinesterase-histochemical observations. Brain Behav Evol. 1996;47:279–310. doi: 10.1159/000113247. [DOI] [PubMed] [Google Scholar]
- Puelles L, Martinez-de-la-Torre M, Bardet S, Rubenstein JLR. Hypothalamus. In: Watson C, Paxinos G, Puelles L, editors. The mouse nervous system. San Diego: Academic; 2012. pp. 221–312. [Google Scholar]
- Quinlan R, Graft M, Mason I, Lumsden A, Kiecker C. Complex and dynamic patterns of Wnt pathway gene expression in the devloping chick forebrain. Neural Dev. 2009;4:35. doi: 10.1186/1749-8104-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendahl H. Embryologische und morphologische Studien über das Zwischen hirn beim Huhn. Acta Zoologica (Stockholm) 1924;5:241–344. [Google Scholar]
- Rubenstein JL, Martinez S, Shimamura K, Puelles L. The embryonic vertebrate forebrain: the prosomeric model. Science. 1994;226:578–580. doi: 10.1126/science.7939711. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–447. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang T, Lemaistre M, Vortkamp A, Rüther U. Expression of the zing finger gene Gli3 is affected in the morphogenetic mouse mutant extra-toes (Xt) Development. 1992;116:799–804. doi: 10.1242/dev.116.3.799. [DOI] [PubMed] [Google Scholar]
- Scholpp S, Lumsden A. Building a bridal chamber: development of the thalamus. Trends Neurosci. 2010;33:373–380. doi: 10.1016/j.tins.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholpp S, Wolf O, Brand M, Lumsden A. Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon. Development. 2006;133:855–864. doi: 10.1242/dev.02248. [DOI] [PubMed] [Google Scholar]
- Scholpp S, Foucher I, Staudt N, Peukert D, Lumsden A, Houart C. Otx1l, Otx2 and Irx1b establish and position the ZLI in the diencephalon. Development. 2007;134:3167–3176. doi: 10.1242/dev.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura K, Hirano S, McMahon AP, Takeichi M. Wnt-1-dependent regulation of local E-cadherin and αN-catenin expression in the embryonic mouse brain. Development. 1994;120:2225–2234.2. doi: 10.1242/dev.120.8.2225. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Hartigan DJ, Martinez S, Puelles L, Rubenstein JL. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- Tendeng C, Houart C. Cloning and embryonic expression of five distinct sfrp genes in the zebrafish Danio rerio. Gene Exp Patterns. 2006;6:761–771. doi: 10.1016/j.modgep.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Tossell K, Kiecker C, Wizenmann A, Lang E, Irving C. Notch signalling stabilises boundary formation at the midbrain-hindbrain organiser. Development. 2011;138:3745–3757. doi: 10.1242/dev.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa F, Itasaki N, Briscoe J. Inhibitory Gli3 activity negatively regulates Wnt/beta-catenin signaling. Curr Biol. 2007;17:545–550. doi: 10.1016/j.cub.2007.01.062. [DOI] [PubMed] [Google Scholar]
- Vieira C, Martinez S. Experimental study of MAP kinase phosphatase-3 (Mkp3) expression in the chick neural tube in relation to Fgf8 activity. Brain Res Rev. 2005;49:158–166. doi: 10.1016/j.brainresrev.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Vieira C, Martinez S. Sonic hedgehog from the basal plate and the zona limitans intrathalamica exhibits differential activity on diencephalic molecular regionalization and nuclear structure. Neuroscience. 2006;143:129–140. doi: 10.1016/j.neuroscience.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Vieira C, Garda AL, Shimamura K, Martinez S. Thalamic development induced by Shh in the chick embryo. Dev Biol. 2005;284:351–363. doi: 10.1016/j.ydbio.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Vieira C, Pombero A, Garcia-Lopez R, Gimeno L, Echevarria D, Martinez S. Molecular mechanisms controlling brain development: an overview of neuroepithelial secondary orgnizers. Int J Dev Biol. 2010;54:7–20. doi: 10.1387/ijdb.092853cv. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nakamura H. Control of chick tectum territory along dorsoventral axis by Sonic hedgehog. Development. 2000;127:1131–1140. doi: 10.1242/dev.127.5.1131. [DOI] [PubMed] [Google Scholar]
- Zeltser LM. Shh-dependent formation of the ZLI is opposed by signals from the dorsal diencephalon. Development. 2005;132:2023–2033. doi: 10.1242/dev.01783. [DOI] [PubMed] [Google Scholar]
- Zeltser LM, Larsen CW, Lumsden A. A new developmental compartment in the forebrain regulated by Lunatic fringe. Nat Neurosci. 2001;4:683–684. doi: 10.1038/89455. [DOI] [PubMed] [Google Scholar]
- Zhou CJ, Pinson KI, Pleasure SJ. Severe defects in dorsal thalamic development in low density lipoprotein receptor-related protein-6 mutants. J Neurosci. 2004;24:7632–7639. doi: 10.1523/JNEUROSCI.2123-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]