Figure 2.
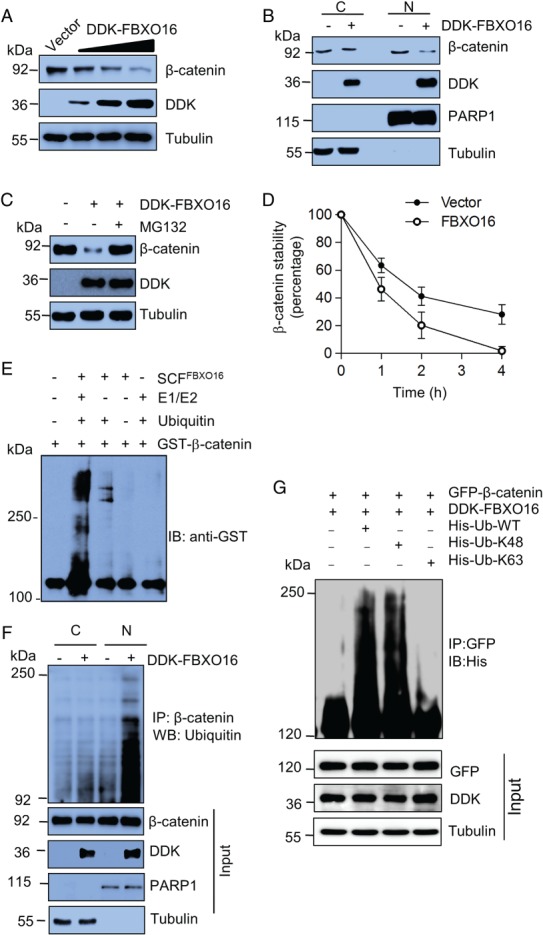
FBXO16 promotes proteasomal degradation of β‐catenin. (A) Ectopically expressed DDK‐FBXO16 decreased the levels of β‐catenin in a dose‐dependent manner. Whole‐cell lysates of MDA‐MB‐231 cells expressing either vector control or increasing doses of DDK‐FBXO16 were immunoblotted with the indicated antibodies (n = 3). MDA‐MB‐231 cells were transfected using Lipofectamine 3000 according to the manufacturer's protocol throughout our study. (B) Fractionated cell lysates of MDA‐MB‐231 cells expressing either vector control or DDK‐FBXO16 were immunoblotted using the indicated antibodies. PARP1 and tubulin were used as nuclear and cytoplasmic loading controls, respectively (n = 3). (C) MDA‐MB‐231 cells were transfected with either vector control or DDK‐FBXO16 for 42 h. FBXO16‐transfected cells were then incubated with or without 5 μm MG132 for 6 h. Whole‐cell lysates were immunoblotted for the indicated proteins (n = 4). (D) Levels of β‐catenin (in cycloheximide assay, see supplementary material, Figure S2C) were quantified and normalized to the loading control. Expression levels of β‐catenin were then normalized to 100% at time 0 h. (E) In vitro ubiquitylation assay showed that FBXO16 promotes ubiquitylation of β‐catenin. Purified FBXO16, GST‐ β‐catenin, ubiquitin, and E1, E2 enzymes were incubated together and immunoblotted for anti‐GST antibody. (F) Cytoplasmic (C) and nuclear (N) protein extracts of MDA‐MB‐231 cells expressing either vector control or DDK‐FBXO16 were immunoprecipitated with β‐catenin. Immunoprecipitates and input protein extracts were immunoblotted for indicated proteins. Cells were treated with MG132 (10 μm for 4 h) prior to lysis. PARP1 and tubulin were used as nuclear and cytoplasmic loading controls, respectively (n = 2). (G) FBXO16 promotes K48‐linked polyubiquitylation of β‐catenin. Whole‐cell lysates of MCF7 cells coexpressing GFP‐β‐catenin and DD‐FBXO16 along with HIS‐K63/HIS‐K48 Ub for 48 h were immunoprecipitated with anti‐GFP antibody, followed by immunoblotting for His. Whole‐cell lysates were immunoblotted for the indicated proteins. The experiment was repeated three times.
