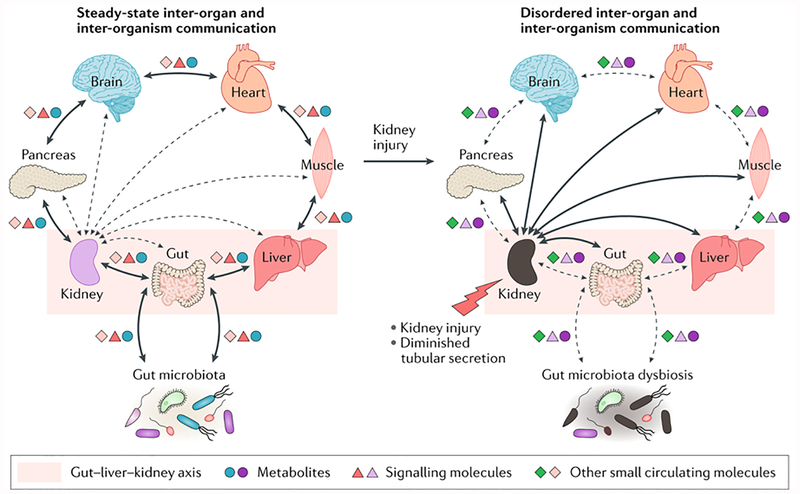
Figure 1. Aberrant inter-organ and inter-organism communication contributes to uremic syndrome.
According to the remote sensing and signaling hypothesis, illustrated here from the perspective of the kidney, steady-state communication between the kidney and other organs and body fluids involves the movement of metabolites, signaling molecules and other small circulating molecules via solute carrier (SLC) and ATP-binding cassette (ABC) transporters. These transporters are expressed in many tissues, including the kidney, liver, pancreas, brain, intestine and muscle. Therefore, injury to the kidney leads to diminished tubular secretion and results in disordered remote communication. Some of these molecules can regulate the expression (via nuclear receptor activation) and/or function of SLC and ABC transporters or phase 1 and phase 2 drug-metabolizing enzymes (DMEs) in distinct cells, tissues and organs, thereby effecting local and global physiological alterations, accompanied by dysbiosis of gut microbiota (including the loss or gain of some bacterial strains). The multi-specificity of some ABC and SLC transporters, together with their different and modifiable tissue expression and/or trafficking, might help to restore homeostasis after organ dysfunction and injury. The differences in color of the symbols representing metabolites, signaling molecules and other small circulating molecules that interact with transporters represent alterations in the concentration and/or identity of these compounds under physiological versus pathological conditions.