Figure 2.
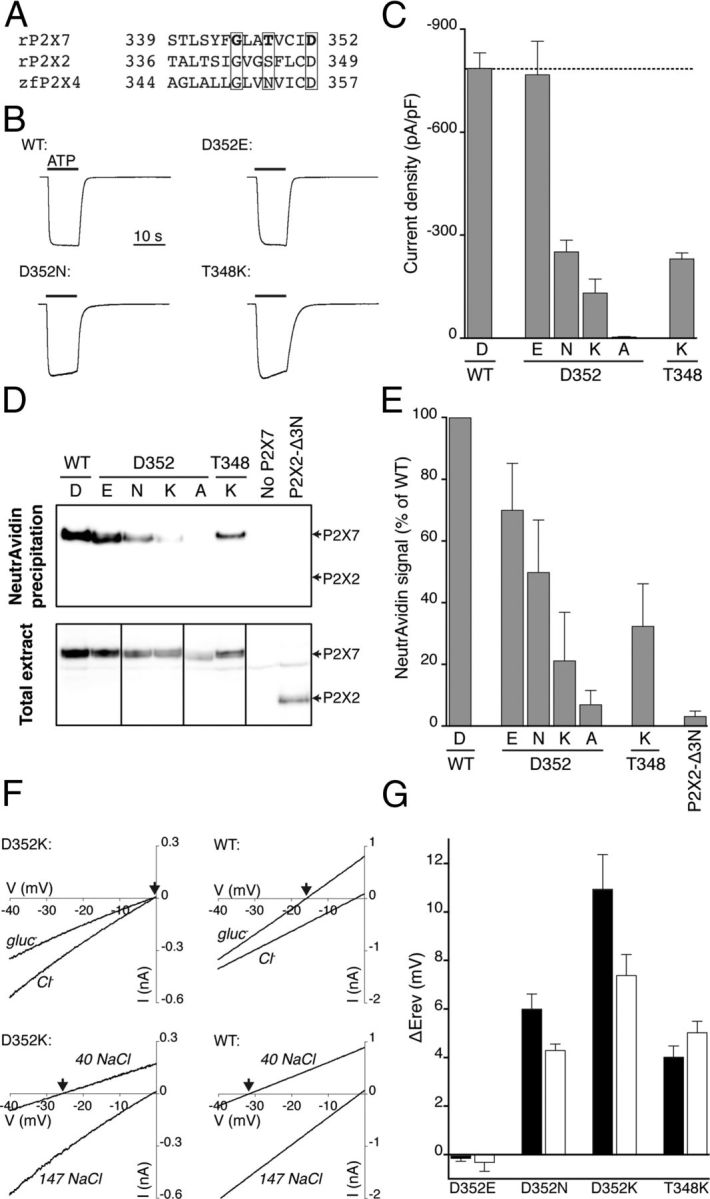
Pore mutations increase permeability to chloride ions. A, Aligned sequences of part of the second transmembrane domain of rat P2X7, rat P2X2 and zebrafish P2X4 receptors. The boxed residues are those changed in the present study (rat P2X7 G345, T348, and D352). B, Currents elicited by ATP (5 mm) at wild-type and mutated P2X7 receptor (normalized: amplitudes were wild-type 10.4 nA, D352E 7.2 nA, D352N 2.3 nA, and T348K 3.1 nA). Error bars indicate the SE of the mean for 8–28 cells. C, Peak current densities for ATP-evoked currents in wild-type and mutated P2X7 receptors. D, Surface expression of wild-type and mutated P2X7 receptors. E, Band densities were measured from three independent biotinylation experiments, one of which is illustrated in D. F, Representative current–voltage relationships for wild-type P2X7 receptors and mutation D352K. Voltage ramps were delivered in the presence of agonist in control solution (see Materials and Methods) and a solution in which most extracellular chloride ions were substituted by gluconate (top; gluc−), or most extracellular sodium and chloride was substituted by mannitol (bottom; 40 NaCl). In both cases, wild-type and mutant P2X7 receptors show different reversal potentials (arrows) in reduced extracellular chloride concentrations. G, The difference in reversal potential compared with wild-type P2X7 receptors. These plots are uncorrected for liquid junction potentials. Open bars represent mannitol substitution. Filled bars represent gluconate substitution.
