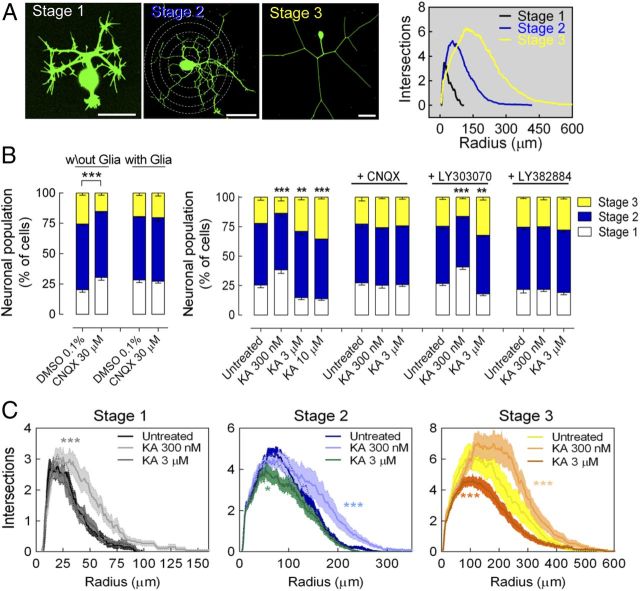
Figure 2.
KARs bidirectionally modulate neuronal maturation and neuritic morphology in DRG neurons. A, Representative images of DRG neurons 20 h after infection with a Sindbis virus encoding GFP, showing neurons classified according to morphological criteria: immature stage 1 neurons, intermediate stage 2 neurons, and mature stage 3 neurons. Scale bars, 50 μm. Neurons at each stage of maturation exhibit distinguishable characteristics and branching profiles, as determined by Sholl analysis (graph, right). B, In the absence of glial cells, the selective antagonist of AMPAR/KAR CNQX (30 μm) delayed neuronal maturation. Left: In neurons cultured with glia, the tonic activation of KARs was prevented, as indicated by the absence of effect of CNQX. Right: Bidirectional effect of KA on the maturation of GFP+-DRG neurons. KA delays maturation at low concentrations, promoting maturation at higher concentrations. This biphasic effect is prevented by the selective AMPAR/KAR antagonist CNQX (30 μm) and by LY382884 (10 μm), an antagonist of GluK1-containing KARs, yet not by the selective AMPAR antagonist LY303070 (25 μm). Data are expressed as the percentage of neurons (mean ± SEM) at each stage of maturation quantified from 4–5 different cultures with a minimum of 100 neurons analyzed per condition and culture. **p < 0.01 and ***p < 0.001, two-way ANOVA with Bonferroni's post hoc test for comparisons versus control conditions. C, Bidirectional modulation of the neuritic morphology of DRG neurons (Sholl analysis) after exposure to KA. The data are expressed as the number of intersections (mean ± SEM) in 1 μm incremental radii from the soma quantified from four different cultures analyzing 25–50 neurons per stage, condition, and culture: *p < 0.05 and ***p < 0.001, two-way ANOVA.