Figure 1.
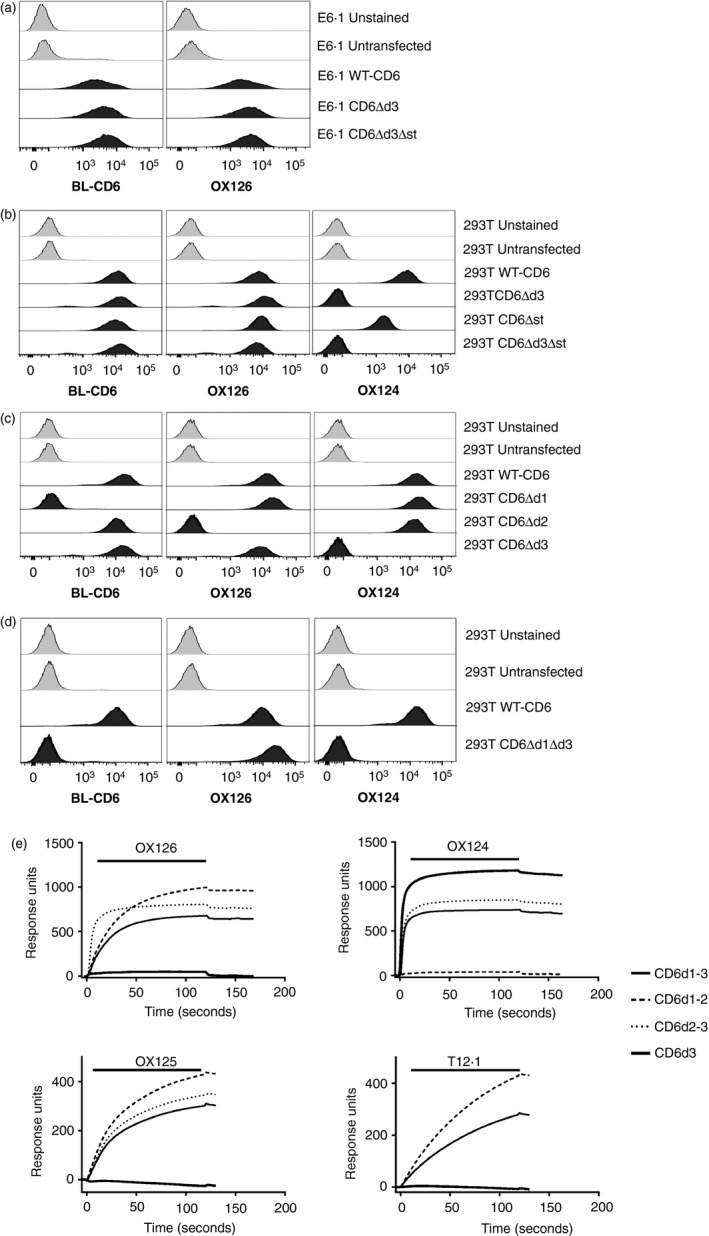
CD6 monoclonal antibodies (mAbs) OX126 and OX124 bind domain 2 (d2) and d3, respectively. Flow cytometry analysis of (a) Jurkat E6.1 and (b–d) HEK293T cells transfected with wild‐type (WT) ‐CD6 and domain deletion (Δ) mutants using CD6d1 mAb (BL‐CD6), OX126 and OX124. (a) OX126 binding does not depend on d3 and/or the membrane proximal stalk region of CD6. (b) OX124 binding depends on d3. (c) OX126 binding depends on d2. (d) OX126 binds directly to d2 without a contribution of other domains. (e) CD6 mAbs, OX126, OX124, OX125 and a d1 mAb (T12.1) were injected over the fusion proteins consisting of CD6 domains 1–3 (CD6d1–3), CD6d1–2, CD6d2–3 and CD6d3, immobilized on the surface of a CM5 chip. Sensorgram traces show that mAb binding depends on the presence of d2 for OX126 and OX125; d3 for OX124 and d1 for T12.1.
