Figure 3.
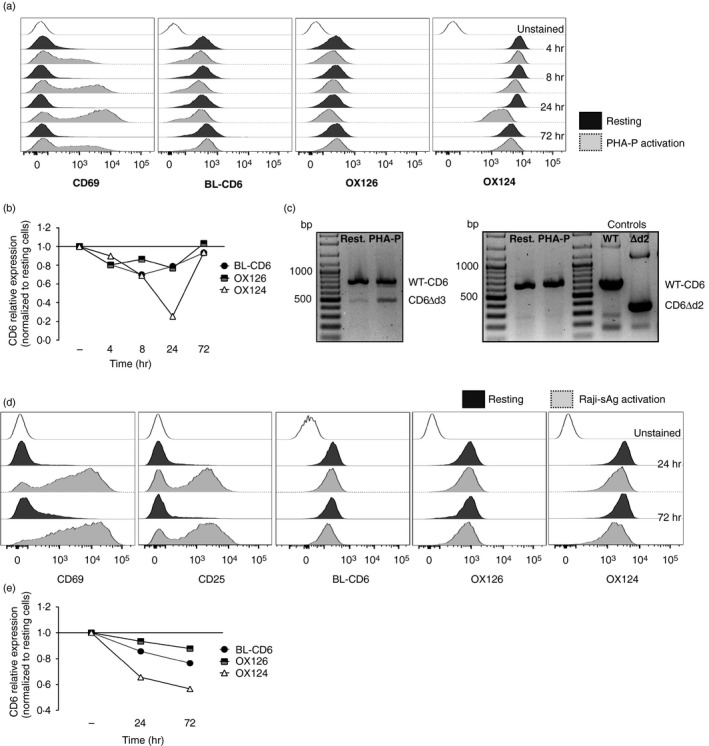
The CD6Δd3 alternative isoform, but not CD6Δd2, is induced upon activation. (a, b) Flow cytometry analysis of resting and phytohaemagglutinin‐P‐activated human peripheral lymphocytes, gated on CD3+ cells, stained with CD69, CD6d1 (BL‐CD6), OX126 and OX124 monoclonal antibodies (mAbs). (a) Expression of the activation marker CD69, and of CD6 domain 1 (d1), d2 and d3 upon stimulation with phytohaemagglutinin‐P for 4, 8, 24 and 72 hr. (b) Graphical representation of the kinetics of expression of CD6 d1, d2 and d3, assessed by normalization of the geometric mean fluorescence intensity of activated to resting cells. The expression of d3 is significantly reduced at 24 hr post activation, coinciding with the peak of CD69 expression shown in (a). (c) mRNA expression of CD6 isoforms in resting and 24 hr‐activated cells corroborates the results of the previous panels, with the increase of a transcript that encodes the CD6Δd3 isoform, which skips the d3‐encoding exon 5. Left panel: polymerase chain reaction amplification using a forward primer on exon 3 (encoding d1) and a reverse primer on exon 7 allowed the identification of a smaller isoform indicative of the skipping of a single domain‐encoding exon. Right panel: complementary polymerase chain reactions were performed to identify which of the exons 4 and 5 is skipped. Using a forward primer on exon 3 (d1) and a reverse primer on exon 5 (d3), transcripts corresponding to wild‐type (WT) ‐CD6, with a predicted size of 660 bp, were obtained from resting and 24‐hr phytohaemagglutinin‐P‐activated human peripheral lymphocytes. However, no mRNA corresponding to the CD6Δd2 isoform, having a predicted size of 348 bp, could be detected. WT‐CD6 and CD6Δd2 cDNAs were used as templates for positive control reactions. (d, e) Flow cytometry analysis and graphical representation of the expression of CD6 d1, d2 and d3 of human peripheral lymphocytes (gated on CD4+ cells) interacting with superantigen‐pulsed Raji cells (Raji‐sAg activation) or with unprimed Raji cells (resting). (d) T cells were stained with mAbs against CD69, CD25 and CD6 d1, d2 and d3 (BL‐CD6, OX126 and OX124, respectively) and the corresponding kinetics normalization (e) was as detailed in (b). Although the decrease of OX124 expression is less pronounced, d3‐mediated splicing is observed at 24 hr and more pronounced at 72 hr.
