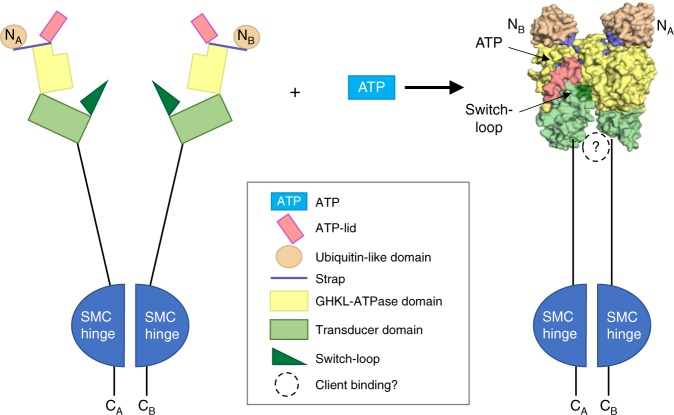
Fig. 6.
Proposed clamp-like closure of SMCHD1 upon ATP binding. Potential conformational changes in SMCHD1 during the transition from apo-monomer to ATP-bound dimer based on similar conformational changes that occur upon dimerization in other GHKL-ATPases. Binding of ATP results in closure of the ATP lid along the ATPase/transducer interface and repositioning of the transducer domain and switch-loop with respect to the ATPase domain; this also coincides with a domain swap of the strap and ubiquitin-like (UBL) domain. In other GHKL-ATPases, dimerization generates a binding cavity at the C-terminal end of the transducer domain. This cavity may serve as a binding site for SMCHD1 binding partners