These data provide insights into the distinctive functions of VdSsk2 and VdSte11 in pathogenicity, stress adaptation, and microsclerotial formation in V. dahliae.
KEYWORDS: MAPKKK, Verticillium dahliae, microsclerotia, pathogenicity, stress response
ABSTRACT
Verticillium dahliae causes destructive vascular wilt diseases on more than 200 plant species, including economically important crops and ornamental trees worldwide. The melanized microsclerotia enable the fungus to survive for years in soil and are crucial for its disease cycle. Previously, we found that the VdPbs2-VdHog1 (V. dahliae Pbs2-V. dahliae Hog1) module plays key roles in microsclerotial formation, stress responses, and virulence in V. dahliae. In this study, two mitogen-activated protein kinase kinase kinases (MAPKKKs) homologous to Ssk2p and Ste11p, which activate the Pbs2p-Hog1p module by phosphorylation in budding yeast, were identified in the genome of V. dahliae. Both ΔVdSsk2 (V. dahliae Ssk2) and ΔVdSte11 strains showed severe defects in microsclerotial formation and melanin biosynthesis, but the relative importance of these two genes in microsclerotial development was different. Deletion of VdSsk2, but not VdSte11, affected responses to osmotic stress, fungicidal response, and cell wall stressors. The ΔVdSsk2 strain exhibited a significant reduction in virulence, while the ΔVdSte11 strain was nonpathogenic due to failure to penetrate and form hyphopodia. Phosphorylation assays demonstrated that VdSsk2, but not VdSte11, can phosphorylate VdHog1 in V. dahliae. Moreover, VdCrz1, encoding a calcineurin-responsive zinc finger transcription factor and a key regulator of calcium signaling in fungi, was misregulated in the ΔVdSsk2, ΔVdPbs2, and ΔVdHog1 mutants.
IMPORTANCE These data provide insights into the distinctive functions of VdSsk2 and VdSte11 in pathogenicity, stress adaptation, and microsclerotial formation in V. dahliae.
INTRODUCTION
Verticillium dahliae is a soilborne fungus that infects more than 200 dicotyledonous plant species and causes devastating vascular wilt diseases (1). The resting structures of this fungus are known as microsclerotia, which generate infectious hyphae that penetrate the host epidermis directly and colonize the root cortex. After a brief period in the cortex, the fungus enters the xylem tissue (2). As vascular colonization and wilt development progresses, V. dahliae produces melanized microsclerotia in dying plant tissues, representing new primary inocula (3). Melanin deposition is tightly coupled with microsclerotial development (4), though it is not required for pathogenicity (5). However, melanin does provide protection from UV irradiation and temperature extremes (5), enzymatic lysis, nutrient deprivation, and fungicidal activities (6). Thus, melanized microsclerotia are vital to the survival of this pathogen in soil, sometimes for very long periods, in the absence of a host plant. Because of their vitally important roles in the life cycle and disease spread, microsclerotia are considered important targets for disease control (4, 7). Therefore, elucidation of the molecular mechanisms that underlie microsclerotial development and pathogenicity, including the key signaling pathways that control these processes, represent important steps in the quest for novel control strategies.
Mitogen-activated protein kinase (MAPK) cascades, as key cores of signaling networks in fungi and other eukaryotes, mediate the signaling required to initiate infection-related morphogenesis and for a variety of cellular adaptations to diverse stimuli (8). To activate the MAPK cascade, a MAPK kinase kinase (MAPKKK) phosphorylates the MAPK kinase (MAPKK), which in turn activates the MAPK by phosphorylation. The high-osmolarity glycerol (Hog) pathway is one of the MAPK signaling pathways that is activated by deleterious environmental conditions, such as high osmolarity, citric acid, and heat stresses (9). This pathway is well understood in the yeast Saccharomyces cerevisiae (9, 10). The canonical Hog pathway contains two branches, mediated by Sho1p and Sln1p, which converge on the MAPKK Pbs2p (11, 12). Two MAPKKKs, Ste11p and Ssk2p/Ssk22p, activate the Pbs2p-Hog1p module in S. cerevisiae (13).
The core components of the Hog pathway are well conserved in fungi, and their biological roles in regulating stress adaptation have been reported in several filamentous plant-pathogenic fungi, such as Magnaporthe oryzae, Cryphonectria parasitica, Botrytis cinerea, Mycosphaerella graminicola, and the oomycete Phytophthora sojae (14–19). Systematic characterization of the kinome of plant pathogen Fusarium graminearum revealed that F. graminearum Ssk22 (FgSsk22) functions upstream of FgPbs2 and FgOs2 (20), indicating that it is different from yeast somehow. However, our knowledge about upstream MAPKK and MAPKKKs in filamentous fungi is more limited.
Calcium (Ca2+) signaling is also involved in fungal development and stress tolerance (21). After binding Ca2+, the protein calmodulin activates calcineurin (22). Crz1 (calcineurin-responsive zinc finger) is an important downstream transcription factor, whose function is dependent on appropriate Ca2+ signaling in fungi (23). For example, M. oryzae CRZ1 (MoCRZ1) is essential for tolerance to ion stress, maintaining cell wall integrity, and virulence in M. oryzae (24, 25). In B. cinerea, the CRZ1 homolog is required for sclerotial formation and pathogenicity (26).
In recent years, some of the key components of signal transduction in V. dahliae have been characterized, including those of MAPK pathways (VMK1, VdMsb, VdSho1, VdPbs2, VdHog1, and VdMsn2) (27–31), and VdCrz1 (32). Vmk1 was the first characterized MAPK in V. dahliae, and it is an ortholog of yeast Kss1p, which is required for filamentous growth in S. cerevisiae (33). Vmk1 is essential for pathogenicity, though the vmk1 mutant is also defective in conidiation and microsclerotial formation (31). V. dahliae mutants lacking the transmembrane mucin, VdMsb, exhibit significant reductions in invasive growth, adhesive capacity, conidiation, and microsclerotial formation (34). VdSho1 controls external sensing, virulence, and multiple growth-related traits in V. dahliae (30). Previously, the kinase module VdPbs2-VdHog1 has been functionally characterized in V. dahliae (28, 29). Yet the roles of upstream MAPKKKs VdSsk2 and VdSte11, which are conserved and thought to signal to the Hog MAP kinase cascade, are not well understood in V. dahliae.
The objective of this current study was to confirm or establish functional similarities in signaling, if any, between VdSsk2 and VdSte11, as upstream MAPKKKs, and secondarily to determine whether these kinases contribute to virulence in V. dahliae. In this study, we have characterized the roles of VdSsk2 and VdSte11 in signal transduction in V. dahliae. Overall, our data indicate that V. dahliae differs from the model yeasts in the lack of convergence of both Ssk2p and Ste11p on the Hog pathway, and this supports a model whereby only VdSsk2 acts upstream of the VdPbs2-VdHog1 module. VdSsk2 is a major regulator of some stress responses analyzed, and VdSte11 is essential for pathogenicity and melanin biosynthesis. Through genetic analyses, we discovered that VdSsk2 and VdSte11 have distinctive functions in microsclerotial formation and that the calcineurin-responsive zinc finger transcription factor VdCrz1 acts downstream of the Hog MAPK pathway through Ca2+ signaling. These findings clarify functional relationships of key upstream components of signaling pathways that govern virulence, microsclerotial development, and stress responses in V. dahliae.
RESULTS
VdSte11 affects hyphal morphology in V. dahliae.
MAPKKKs homologous to yeast Ssk2/Ssk22p and Ste11p were identified in the genome of V. dahliae. The two genes, VdSsk2 (V. dahliae Ssk2) and VdSte11, encode proteins that share high identity to the yeast proteins Ssk2p and Ste11p, respectively. The yeast has two kinases, Ssk2p and Ssk22p, whereas V. dahliae has only VdSsk2. VdSsk2 and VdSte11 are also highly homologous to other Ssk2p and Ste11p orthologs from other fungi, respectively (see Fig. S1 and S2 in the supplemental material). To functionally investigate the roles of VdSsk2 and VdSte11, three independent deletion mutants were obtained for each gene and verified by multiple PCR analyses and DNA blots (Fig. S3). Mutants were complemented by introduction of the wild-type copies of either VdSsk2 or VdSte11 into the mutant strains (Fig. S3).
Sequence analyses of VdSsk2 from Verticillium dahliae and its orthologs. (a) Schematic charts of putative conserved domains encoded by VdSsk2 compared with S. cerevisiae Ssk2p. STKc_MEKK4 domains are shown in boxes. (b) Unrooted neighbor-joining tree of VdSsk2 protein was constructed with its orthologs from other fungi. The phylogenetic tree was constructed using MEGA 6.0 (62) using the full-length protein sequences. Bootstrap values (as a percentage) obtained with 1,000 replicates are indicated on branches, and branch lengths correspond to genetic distance (the scale bar at the lower left corresponds to a genetic distance of 0.1). The amino acid sequences include VDAG_08787 and its homologs from Saccharomyces cerevisiae (ScSsk22, YCR073C; ScSSK2, YNR031C), Verticillium alfalfae (VDBG_04429), Metarhizium robertsii (MAA_04290), Neurospora crassa (NCU03071), Fusarium oxysporum (FOXG_00643), Magnaporthe oryzae (MGG_00183), Botrytis cinerea (BC1G_04606), Ustilago maydis (UMAG_01544), Cryptococcus neoformans (CNL05560), Aspergillus niger (ANI_1_450074), Candida albicans (CAALFM_C405030CA), Beauveria bassiana (EJP70068), and Metarhizium robertsii (XP_007820479). Download FIG S1, TIF file, 2.4 MB (2.5MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Sequence analyses of VdSte11 from Verticillium dahliae and its orthologs. (a) Schematic charts of conserved protein domains encoded by VdSte11 compared with S. cerevisiae Ste11p. Domains, including SAM, Ras_bdg_2, and PKc_like superfamily are shown in boxes of different colors. (b) Unrooted neighbor-joining tree of VdSte11 protein was constructed with its orthologs from other fungi. A phylogenetic tree was constructed using MEGA 6.0 (62) using the full-length protein sequences. Bootstrap values (as a percentage) obtained with 1,000 replicates are indicated on branches, and branch lengths correspond to genetic distance (a scale bar at the lower left corresponds to a genetic distance of 0.1). The amino acid sequences include VDAG_05822 and its homologs from S. cerevisiae (YLR362W), V. alfalfae (VDBG_06755), F. oxysporum (FOXG_09411), M. oryzae (MGG_14847), N. crassa (NCU06182), M. robertsii (MAA_03529), B. bassiana (EJP69245.1), B. cinerea (ACJ06645.1), A. niger (XP_001398357.2), A. nidulans (AN2269.2), and C. albicans (C203770CA). Download FIG S2, TIF file, 2.4 MB (2.5MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Deletion of VdSsk2 and VdSte11 in Verticillium dahliae. (a) Schematic diagram of construction of gene deletion cassettes using the split marker method (64). (b) Confirmation of the VdSte11 gene deletion by PCR. Following PCR amplification with the internal screening primer pairs (RT-primer) LE13/LE14 and the specific primer pairs LE9/LE14, a 450-bp band and a 1,943-bp band were detected for the wild-type strain and the complementary strains of VdSte11 gene, but no band showed in the transformant e16. A 1,700-bp band was amplified with the universal primer M13F/M13R from the genomic DNA of the transformant, e16. (c) Confirmation of the VdSsk2 gene deletion mutant by PCR. Using the internal screening primer pairs, LY190/LY191, a 318-bp band was amplified from genomic DNA from the wild-type strain XS11 and the complemented strains of VdSsk2 gene, but no band was found in ΔVdSsk2-2 ΔVdSsk2-50 strains. (d) The internal screening primer pairs LE13/LE14 and LY190/LY191 were used to confirm VdSte11 and VdSsk2 gene complementation with cDNA by reverse transcription-PCR, respectively. Primer pairs VdBt-up/VdBt-down were used to verify the quality of the genomic DNA and cDNA of all strains in this assay. Download FIG S3, TIF file, 1.8 MB (1.9MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
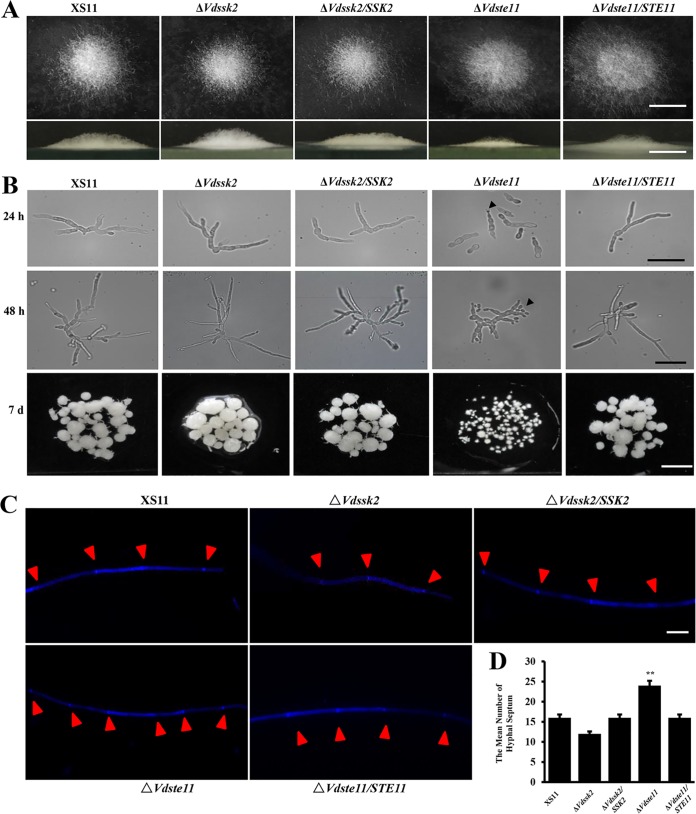
Phenotypic observations of the ΔVdSsk2 and ΔVdSte11 strains revealed no major growth defects on various media (Fig. S4). However, the colonies of the ΔVdste11 strain appeared smooth and exhibited less aerial mycelia than the colonies of other strains (Fig. S4). After incubation on basal medium (BM), aerial mycelial growth was markedly reduced in the ΔVdSte11 strain versus the ΔVdSsk2 strain, the complemented strains, and wild-type strain XS11 (Fig. 1A). The germ tubes from the conidia of the ΔVdSte11 strain were convoluted with disproportionate branching and septation relative to the other strains, appearing near yeast-like in growth at 24 and 48 h postinoculation (hpi). Furthermore, the mycelial clumps of the ΔVdSte11 strain that formed in the liquid 7 days postinoculation (dpi) were strikingly smaller than the wild-type strain XS11 and the ΔVdSsk2 strain (Fig. 1B). After staining with calcofluor white (CFW), the ΔVdSte11 strain exhibited enhanced hyphal compartmentalization relative to other strains (Fig. 1C, red arrowheads). The mean number of hyphal septa of the ΔVdSte11 strain was 1.5 times more than that of other strains (Fig. 1D). Given these findings above, we concluded that VdSte11 plays an essential role in filamentous growth over solid surfaces and in liquid media, while VdSsk2 has no obvious effect on filamentous growth and morphology.
FIG 1.
The phenotypes of ΔVdSsk2 and ΔVdSte11 strains of Verticillium dahliae in axenic culture. (A, top) Colony morphology of XS11, ΔVdSsk2, and ΔVdSte11 strains, and the respective complemented strains were incubated on BM for 7 days. The aerial hyphal growth of the strains was observed after growth. (Bottom) Vertical dissection of the colonies. Bars = 1 cm. (B) Strains were cultured in yeast extract peptone-dextrose liquid medium at 25°C for 24 h, 48 h, and 7 days (d). Germination of conidia and filamentous branching were imaged with a compound microscope. All cultures were inoculated with the same amount of spores (106 conidia/ml). Bars = 0.5 cm. (C) Strains were cultured on PDA for 7 days. Germ tubes from germinated conidia and hyphae were stained with 10 mg/ml CFW for 5 min. The CFW signal was imaged by fluorescence microscopy, and the CFW signal in the hyphal septum was quantified separately. Bar = 10 μm. (D) Number of hyphal septum calculated in the single hypha in panel C. Values are means plus standard deviations (error bars) from three replicates. Values that are significantly different (P < 0.01) from the value for the wild-type strain XS11 are indicated by two asterisks.
Colony morphology in the wild-type (XS11), ΔVdSte11, and ΔVdSsk2 strains. Colony morphology of XS11, ΔVdSsk2, and ΔVdSte11 strains, and the respective complemented strains were incubated on CM and PDA for 7 days. Bar indicates 1 cm. Download FIG S4, TIF file, 2.3 MB (2.3MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
VdSsk2 and VdSte11 play distinct roles in microsclerotial formation and melanin biosynthesis.
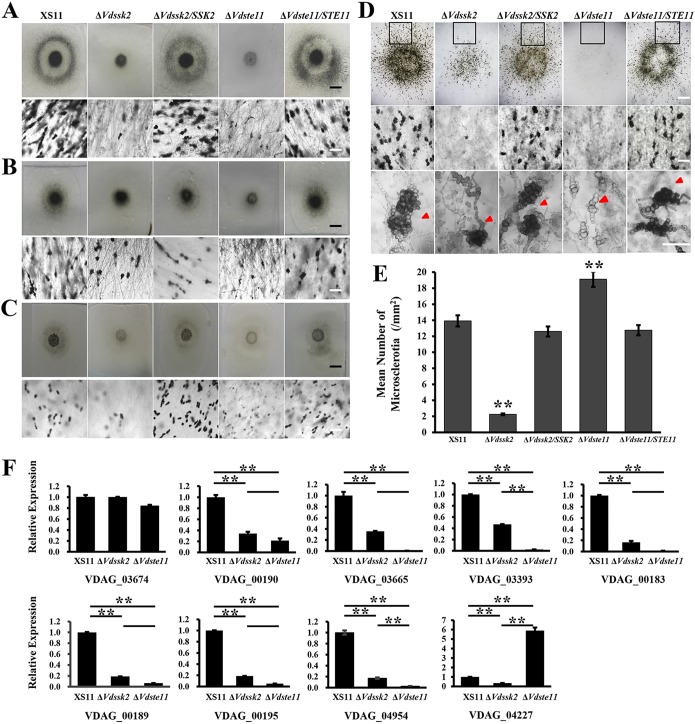
To investigate functions of VdSsk2 and VdSte11 in microsclerotial formation, we first assessed the ability of the above strains to form microsclerotia on different media, such as potato dextrose agar medium (PDA), BM, and complete medium (CM). Microscopic examination showed that the ΔVdSte11 andΔVdSsk2 strains produced less melanized microsclerotia compared to wild-type strain XS11 and the complemented strains (Fig. 2A, B, and C). Interestingly, deletion of VdSte11 led to more severe defect in melanization. Further observations and quantification demonstrated that the ΔVdSsk2 strain produced melanized microsclerotia in significantly reduced numbers (five times less than strain XS11), while the ΔVdste11 strain produced smaller microsclerotia that were devoid of the pigmentation characteristic of melanin deposition (Fig. 2D). Strikingly, swollen hyphal clusters (microsclerotial initials) abundantly appeared amid the colonies of the ΔVdste1 strain. Quantification of microsclerotia revealed that the ΔVdste11 strain produced clusters about 1.3 times more than strain XS11 did (Fig. 2E). Even at 60 dpi, while the ΔVdSsk2 strain formed similar sized melanized microsclerotia similar to those observed in strain XS11, the ΔVdste11 strain produced fewer melanized microsclerotia, which were also smaller than those of the other strains examined (Fig. S5). These results indicated that both VdSsk2 and VdSte11 participate in microsclerotium formation, but the roles of the two are distinctive. That is, VdSsk2 affects the number of melanized microsclerotia, while VdSte11 regulates melanin production or deposition during microsclerotial development.
FIG 2.
Distinct roles of VdSsk2 and VdSte11 in the formation of melanized microsclerotia in Verticillium dahliae. (A to C) Microscopic examination of the colonies of XS11, ΔVdSsk2, and ΔVdSte11 strains and complemented strains cultured on PDA (A), BM (B), and CM (C) at 25°C for 7 days. The top panels show colonies observed with a dissection microscope (bar = 0.5 cm). The bottom panels represent melanized microsclerotial formation with a dissection microscope (bar = 40 μm). (D) The microsclerotial formation of the above strains in detail was observed after the strains were cultured on BM at 25°C for 10 days. The top panels represent the whole view of microsclerotial formation (bar = 0.5 cm). The middle and bottom panels show microsclerotial formation of the black squares in the top panel in more enlarged views at different magnifications (bars = 40 μm). Images were taken by using a microscope. Red arrowheads indicate immature or melanized microsclerotia. (E) Mean number of microsclerotia or hyphal cell aggregation calculated from the black squares in the top panels of Fig. 2B. Error bars represent standard deviations of three replicates, and asterisks represent significant differences (P < 0.01). (F) Relative expression level of nine known genes related with melanin biosynthesis in V. dahliae. RNAs were extracted from the mycelia incubated on PDA for 7 days at 25°C. Error bars represent standard deviations of three replicates, and asterisks represent significant differences (P < 0.01). There were two biological experiments performed for each strain and three technical replicates per biological experiment.
Microsclerotial formation in the wild type (WT), ΔVdSte11, and ΔVdSsk2 strains at 60 dpi. Melanized microsclerotial formation of the strains was observed on the cellulose membranes incubated at 25°C for 60 days. Microscopy was used to assess microsclerotial formation. Download FIG S5, TIF file, 2.5 MB (2.6MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
To determine how VdSsk2 and VdSte11 affect the expression patterns of genes with known roles in melanin biosynthesis or those previously shown as highly upregulated during melanin biosynthesis (4, 5, 7). Reverse transcription-quantitative PCR (RT-qPCR) analyses were performed on the gene sets (Table 1): VDAG_03674, VdPks1 (VDAG_00190), VdBrn1 (VDAG_03665), VdScd1 (VDAG_03393), VdBrn2 (VDAG_00183), VdLac1 (VDAG_00189), VdCmr1 (VDAG_00195), Vayg1 (VDAG_04954), and VdMsn2 (VDAG_04227). Consistent with a deficiency in melanization during culturing, gene expression data showed that seven genes, including VdPKS1, VdLac1, and VdCmr1, were significantly downregulated in both the VdSsk2 and VdSte11 mutants (Fig. 2F), suggesting that both VdSsk2 and VdSte11 positively regulate the expression of genes involved in melanin biosynthesis. However, the expression levels of these genes in the ΔVdste11 strain were much lower than that of the ΔVdSsk2 mutant (Fig. 2F), indicating that VdSte11 functions as a major regulator of melanin biosynthesis and is further consistent with the phenotype of the ΔVdSte11 strain. However, the transcript levels of VDAG_03674 observed in both mutants were not significantly different from those of strain XS11. The expression of VdMsn2 (VDAG_04227), which is a negative regulator of melanized microsclerotial formation (27), was obviously downregulated in the ΔVdSsk2 strain but significantly upregulated in the ΔVdSte11 strain. This strengthened the idea that VdSte11 might play a major role in melanin biosynthesis in V. dahliae. Collectively, we concluded that VdSsk2 and VdSte11 have overlapping functions in regulating some melanin biosynthetic genes, but their roles are different in melanin biosynthesis and microsclerotial development, suggesting the participation in different signaling pathways in V. dahliae.
TABLE 1.
Known and putative melanin biosynthetic genes of Verticillium dahliaea
| Gene ID/name in V. dahliae | Function | Reference |
|---|---|---|
| VDAG_03674 | Acetyl-CoA carboxylase | 4 |
| VDAG_00190/VdPks1 | Polyketide synthase | 4 |
| VDAG_03665/VdBrn1 | Tetrahydroxynaphthalene reductase | 4 |
| VDAG_03393/VdScd1 | Scytalone dehydratase | 4 |
| VDAG_00183/VdBrn2 | Versicolorin reductase | 4 |
| VDAG_00189/VdLac1 | Laccase | 5 |
| VDAG_00195/VdCmr1 | Transcription factor Pig1 | 5 |
| VDAG_04954/Vayg1 | Polyketide chain shortening | 67 |
| VDAG_04227/VdMsn2 | C2H2 transcription factor | 27 |
Names and functions of the genes tested in qPCRs. CoA, coenzyme A.
Deletion of VdSte11 impairs penetration, whereas deletion of VdSsk2 affects fungal proliferation.
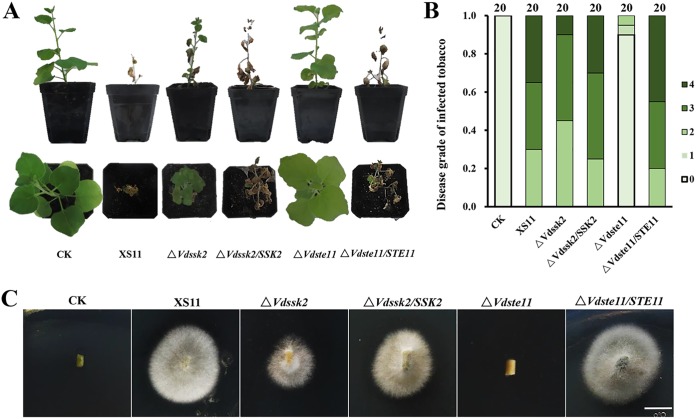
To determine the roles of VdSsk2 and VdSte11 in virulence, we performed pathogenicity assays on tobacco seedlings by root dip inoculation with conidial suspensions (2 × 106 conidia/ml) from each mutant strain, complemented strains, and wild-type strain XS11. At 35 dpi, pathogenicity assays showed that tobacco seedlings inoculated by the ΔVdSte11 strain exhibited no chlorosis, while about 40% of seedlings inoculated by the ΔVdSsk2 strain appeared slightly chlorotic (the yellowing of the leaves in Fig. 3A). In contrast, about 40% of seedlings inoculated with strain XS11 and complemented strains showed severe wilt symptoms (Fig. 3A). Both ΔVdSsk2 and ΔVdSte11 mutants displayed significantly reduced disease severity. Figure 3B shows that the ΔVdSte11 strain was nonpathogenic, and while the ΔVdSsk2 strain caused typical Verticillium wilt symptoms, the disease severity (2 seedlings died, 9 seedlings wilted, and 9 seedlings showed no symptom; n = 20 seedlings) was significantly lower than that observed following inoculation with strain XS11 and the complemented strains (Fig. 3B). Correspondingly, fungal hyphae were not recovered from plants inoculated with the ΔVdSte11 strain, while the ΔVdSsk2 strain could be reisolated from vascular tissue at a lower rate of isolation than those of the VdSsk2-complemented strain and XS11 (Fig. 3C).
FIG 3.
The Verticillium dahliae ΔVdSsk2 and ΔVdSte11 strains exhibit severe defects in pathogenicity. (A) Two-month-old tobacco seedlings were inoculated with a conidial suspension (106 conidia/ml) of XS11 strain, ΔVdSsk2 and ΔVdSte11 deletion strains, and complemented strains for 30 min. Control inoculations (CK) were performed by dipping seedlings in distilled water for 30 min. All seedlings were replanted into the soil after root dipping. Photographs were taken at 35 dpi, respectively. (B) Disease levels were scored according to the number of seedlings that showed wilt symptoms or had died. Twenty seedlings were inoculated with each strain. The disease ratings of the symptoms were calculated at 35 dpi. 0 = no wilting, 1 = yellowing or wilting of <2 leaves, 2 = yellowing or wilting of one-fourth leaves, 3 = yellowing or wilting of two-thirds leaves, and 4 = more than 85% of the leaves wilted or the whole plant died. (C) Reisolation of V. dahliae strains from the system of inoculated tobacco plants at 25°C for 7 days. Images were taken after transferring fungal colonies from the stem sections to PDA at 25°C for 10 days. Bar indicates 0.5 cm.
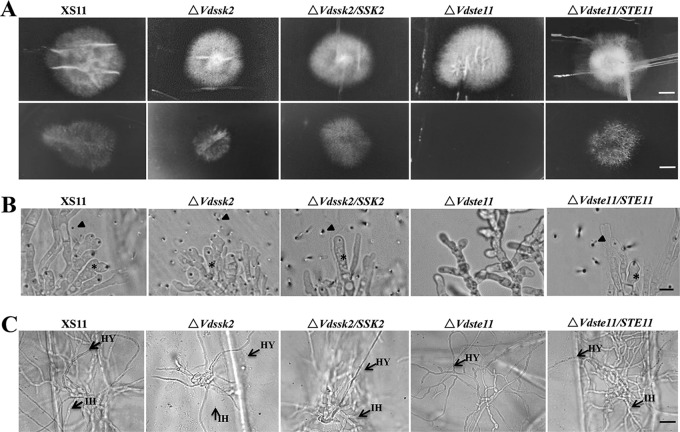
To further characterize the defect of the ΔVdSte11 strain in pathogenicity, we first assayed the penetration of hyphae through the cellophane membrane. On CM overlaid with the cellophane membrane, neither ΔVdSte11 nor ΔVdSsk2 strains exhibited obvious defects in colony morphology at 5 dpi, similar to earlier observations recorded on PDA. However, the inability of hyphae to penetrate through the cellophane membrane was notable in the ΔVdSte11 strain, while there was only a slight defect in penetration of hyphae in the ΔVdSsk2 strain; successful penetration of the wild type and the complemented strains occurred, as visualized after removal of the cellophane membrane at 2 dpi (Fig. 4A). In addition, the ΔVdSte11 strain failed to form hyphopodia for penetration pegs, whereas the ΔVdSsk2 strain, strain XS11, and the complemented strains produced abundant hyphopodia on the cellophane membrane (Fig. 4B). Another penetration assay was performed using onion epidermal tissue. The results of this assay showed that the ΔVdSte11 strain did not penetrate into onion epidermis, since no invasive hyphae (IH) appeared in the ΔVdSte11 mutant, while infectious hyphae were abundant in ΔVdSsk2, XS11, and the complemented strains (Fig. 4C). These results suggest that VdSsk2 and VdSte11 have distinct roles in pathogenesis. In conclusion, deletion of VdSte11 impairs penetration, whereas deletion of VdSsk2 affects proliferation in the xylem.
FIG 4.
Penetration and plant infection analyses of the VdSsk2 and VdSte11 mutant strains of Verticillium dahliae. (A) Cellophane membranes were plated onto minimal medium and inoculated with conidia harvested from wild-type strain XS11, the ΔVdSsk2 and ΔVdSte11 strains, and the respective complemented strains. Penetration was examined at 5 dpi. The top panels show colonies of the strain grown on the cellophane membranes covered above PDA at 5 dpi. The bottom panels show colonies grown from hyphae penetrating through the membrane at another 2 dpi after removing cellophane membrane. Bars = 0.5 cm. (B) Hyphopodia and penetration pegs formed on the cellophane membrane by the above strains. Black asterisks and arrowheads indicate hyphopodia and penetration pegs, respectively. The ΔVdSte11 strain failed to form hyphopodia and penetrate the membrane. Bar = 5 μm. (C) Infection assays of onion epidermis 48 h after inoculation of strains XS11, ΔVdSsk2, ΔVdSte11, and the respective complemented strains. Black arrows point to invasive hyphae (IH) and hyphae (HY). Bar = 20 μm.
The ΔVdSsk2 mutant phenocopies the ΔVdPbs2 and ΔVdHog1 mutants under various stressors.
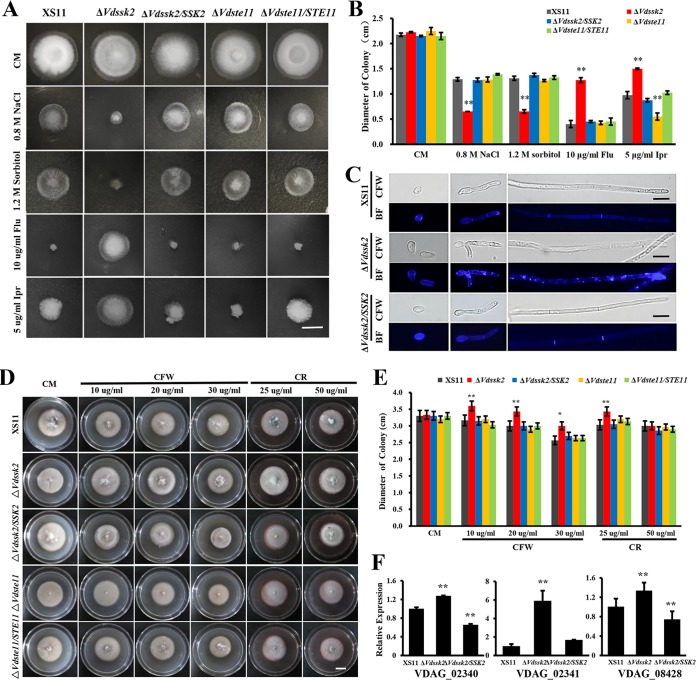
Because deletion of VdPbs2 and VdHog1 led to enhanced sensitivity to osmotic stresses (28, 29), we also examined the ΔVdSsk2 and ΔVdSte11 strains in relation to osmotic stress to determine whether one of these kinases functions in the same pathway as the VdPbs2-VdHog1 module. Compared with wild-type strain XS11 and the complemented strains, the ΔVdSsk2 strain exhibited dramatic hypersensitivity to high-osmolarity stresses such as NaCl and sorbitol and increased resistance to fungicides such as fludioxonil and iprodione (Fig. 5A and B), which was consistent with previous results obtained by examining the ΔVdPbs2 and ΔVdHog1 strains (28, 29). On the other hand, sensitivity of the ΔVdste11 strain to the above stresses was comparable to that observed in strain XS11 (Fig. 5A and B).
FIG 5.
VdSsk2 of Verticillium dahliae is essential to mount the wild-type response to osmotic agents, fungicides, calcofluor white (CFW), and Congo red (CR). (A) The colony morphology of the wild-type strain XS11, the ΔVdSsk2 and ΔVdSte11 strains, and the respective complemented strains grown on complete medium (CM) or CM supplemented with 0.8 M NaCl, 1.2 M sorbitol, 10 μg/ml fludioxonil, and 5 μg/ml iprodione. Photographs were taken at 8 days. Bar = 1 cm. (B) Colony diameters of the indicated strains. Values are means ± standard deviations (error bars) for three replicates, and asterisks show values that are significantly different (P < 0.01). (C) Chitin distribution was observed in strain XS11, the ΔVdSsk2 strain, and the VdSsk2 complemented strain grown in CM liquid supplemented with 0.6 M NaCl for 4 days. Conidia and hyphae were stained with 10 mg/ml CFW for 5 min. The CFW signal was imaged by fluorescence microscopy, and images were also captured by bright-field microscopy (BF). Bars = 10 μm. (D) The colony morphology of the above XS11 strains were grown on CM containing CFW and CR at 25°C for 5 days. Bar = 0.5 cm. (E) Relative growth inhibition of colonies of each strain. Values are means ± standard deviations (error bars) for three replicates, and asterisks show values that are significantly different (P < 0.01). (F) Expression of three genes associated with cell wall biosynthesis was analyzed by RT-qPCR and normalized against the expression of V. dahliae β-tubulin (7). Values are means plus standard deviations (error bars) for three independent experiments, and asterisks show values that are significantly different (P < 0.01).
In addition, we demonstrated that aberrant chitin distribution occurred in the ΔVdSsk2 strain under osmotic stress compared with wild-type strain XS11 (Fig. 5C). The deletion of VdSsk2 caused enhanced growth on media with CFW (10, 20, and 30 μg/ml) and Congo red (CR) (25 μg/ml) compared with strain XS11, respectively (Fig. 5D). The statistical analyses showed that the ΔVdSsk2 strain was significantly different from strain XS11 and the ΔVdSsk2 complementation strains (P < 0.01) (Fig. 5E), but the ΔVdste11 strain showed no difference from strain XS11 (Fig. 5D and E). Moreover, chitin synthase and β-1,3-glucan synthase are involved in the synthesis of chitin and β-1,3-glucan in fungi (35). We identified three genes encoding components involved in cell wall integrity, such as VDAG_02340, VDAG_02341 which encodes 1,3-β-glucan synthase, and VDAG_08428 which encodes a glucan synthesis regulatory protein. Figure 5F shows that loss of VdSsk2 induced significantly higher expression of three genes, indicating that VdSsk2 negatively regulates chitin synthesis. Taken together, the results demonstrated that the deletion of VdSsk2, VdPbs2, and VdHog1, but not VdSte11, caused hypersensitivity to high osmolarity and resistance to fludioxonil, suggesting that VdSsk2 functions in the same signaling pathways as VdPbs2 and VdHog1 in V. dahliae.
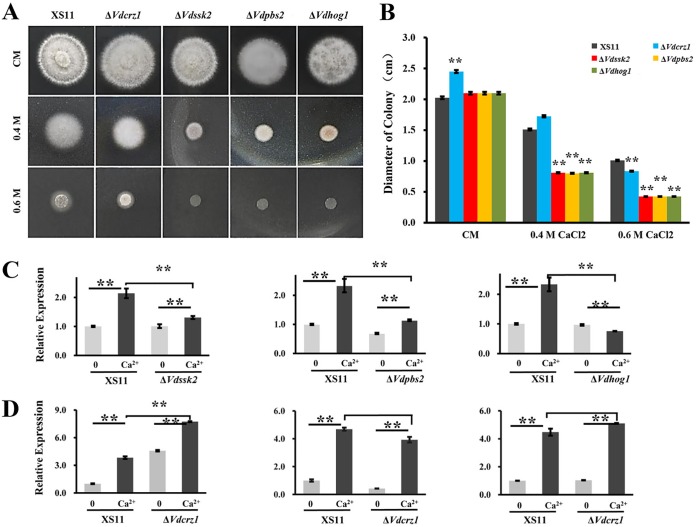
The VdSsk2-VdPbs2-VdHog1 signaling pathway is involved in high Ca2+ concentration tolerance.
To elucidate the contribution of the VdSsk2-VdPbs2-VdHog1 module plus VdSte11 to adaptation of high Ca2+ levels, we tested the tolerance of each mutant to different concentrations of Ca2+. The ΔVdSsk2 strain exhibited increased sensitivity to calcium at higher concentrations, similar to that observed in the ΔVdCrz1, ΔVdPbs2, and ΔVdHog1 strains (Fig. 6A). Interestingly, the ΔVdHog1, ΔVdPbs2, and ΔVdSsk2 strains were more sensitive to higher Ca2+ than the ΔVdcrz1 mutant. The hyphal growth of the ΔVdHog1, ΔVdPbs2, and ΔVdSsk2 strains was approximately 50% of that of the ΔVdcrz1 mutant in the presence of 0.4 M Ca2+ (Fig. 6B). However, the ΔVdSte11 strain showed sensitivity to higher Ca2+ similar to that of strain XS11 (data not shown). Furthermore, we performed expression analyses of VdCrz1 by RT-qPCR to gain insight into the relationship between VdCrz1 expression in the genetic background of the ΔVdHog1, ΔVdPbs2, and ΔVdSsk2 strains and in the wild-type strain XS11. While the expression levels of VdCrz1 were significantly higher in response to Ca2+ in the XS11 strain, the levels of VdCrz1 were not altered in any of the three mutants (Fig. 6C), suggesting that VdCrz1 expression induced by Ca2+ is dependent on the Hog MAPK cascade. In contrast, expression levels of three genes (VdHog1, VdPbs2, and VdSsk2) were upregulated by Ca2+ regardless of the presence or absence of VdCrz1 (Fig. 6D), indicating that the Hog MAPK cascade acts upstream of VdCrz1. These findings indicated that expression of VdCrz1 was at least partially dependent on the VdSsk2-VdPbs2-VdHog1 signaling pathway and that misregulation in the expression of VdCrz1 in response to Ca2+ treatment could account for high Ca2+ sensitivity in the ΔVdHog1, ΔVdPbs2, and ΔVdSsk2 strains.
FIG 6.
VdCrz1 is misregulated in the VdSsk2, VdPbs2, and VdHog1 mutant strains of Verticillium dahliae. (A) Colony morphology of wild-type strain XS11 and of the ΔVdSsk2, ΔVdpbs2, ΔVdhog1, and ΔVdcrz1 strains on CM medium with or without Ca2+. Strains were grown for 5 days. Bar = 1 cm. (B) Colony diameters of strains. Values are means ± standard deviations (error bars) for three replicates, and asterisks show values that are significantly different (P < 0.01). (C) Transcript levels of VdCrz1 were compared between strain XS11 and the ΔVdSsk2, ΔVdpbs2, and ΔVdhog1 strains. Strains were grown on CM without Ca2+ or with 0.3 M Ca2+ for 30 min. Values are means ± standard deviations (error bars) from three independent experiments. (D) The expression levels of VdSsk2, Vdpbs2, and Vdhog1 were analyzed in strain XS11 and the ΔVdcrz1 strain when grown on CM without Ca2+ or with 0.3 M Ca2+ for 30 min. Values are means ± standard deviations (error bars) from three independent experiments.
VdSsk2, but not VdSte11, activates VdHog1.
An initial aim of this work was to investigate whether either the MAPKKKs encoded by VdSsk2 and VdSte11 could phosphorylate VdHog1. Hog1 phosphorylation was not observed in the ΔVdSsk2 strain in this study consistent with observations made on the ΔVdPbs2 strain, but the level of Hog1 phosphorylation in the ΔVdSte11 mutant was similar to that observed in the wild-type strain (XS11) (Fig. 7A). Induction of Hog1 phosphorylation occurred in the ΔVdSte11 strain, and this phosphorylation was stimulated by NaCl, similar to that observed in strain XS11 (Fig. 7B). These findings suggested that VdSsk2, but not VdSte11, functions as the upstream activator of the VdPsb2-VdHog1 module in V. dahliae.
FIG 7.
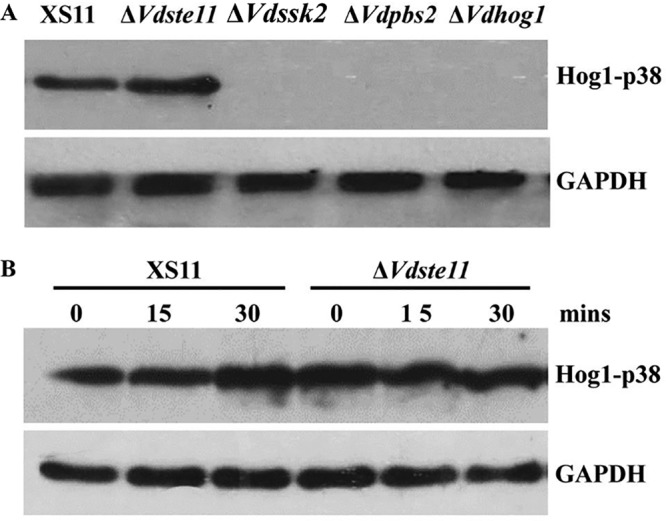
VdHog1 phosphorylation requires VdSsk2, but not VdSte11, in Verticillium dahliae. (A) The phosphorylation levels are shown for VdHog1 in XS11, ΔVdSte11, ΔVdSsk2, ΔVdPbs2, and ΔVdHog1 strains. All strains were incubated for 3 days in YEPD broth, and total proteins were extracted for immunoblot analysis. (B) VdHog1 activation by NaCl induction. The strains were grown for 3 days and exposed to 0.7 M NaCl in YEPD broth for 15 or 30 min, and total proteins were extracted for immunoblot analysis. The separated total proteins from V. dahliae were transferred to a nitrocellulose membrane and the anti-phospho-p38 monoclonal antibody (P-Hog1; Cell Signaling) was used to detect the phosphorylated form of the VdHog1p. The same blots were stripped and then reprobed with GAPDH antibody as an internal control.
DISCUSSION
The Hog MAPK signaling pathway in fungi is important for sensing stressful conditions and the activation of gene expression that enables fungi to resist osmotic stress and to survive under other adverse conditions (13). In S. cerevisiae, the two MAPKKKs, Ste11p and Ssk2/Ssk2p, activate the Pbs2p-Hog1p module. However, whether orthologs of Ste11p and Ssk2/Ssk2p converge on Pbs2 has not been conclusively shown in filamentous fungi (8, 9). Several lines of evidence in this current study demonstrate that VdSsk2, but not VdSte11, functions as an integral component of the Hog signaling pathway in V. dahliae. First, mutants lacking VdSsk2 phenocopied VdPbs2 or VdHog1 deletion mutants in response to stress treatments. Second, as inferred from discernible microsclerotial formation phenotypes, VdSsk2 and VdSte11 play distinctive roles in melanized microsclerotial formation. Third, the phosphorylation level of VdHog1 was affected by the deletion of either VdSsk2 or VdPbs2, but not VdSte11. Finally, VdSte11 inactivation in V. dahliae resulted in the loss of pathogenicity due to a defect in plant infection, while the VdSsk2 deletion resulted in attenuated virulence similar to mutants of VdPbs2 or VdHog1.
The data presented herein suggest that the Ste11p MAPKKK ortholog in V. dahliae does not activate the Ssk2-Pbs2-Hog1 cascade. This is different from the pattern observed in S. cerevisiae, where the Hog1p MAPK is regulated by three functionally redundant MAPKKKs, including Ste11p, Ssk2p, and Ssk2p. In this study, we identified only one kinase (VdSsk2) unlike the two kinases (Ssk2p, and Ssk2p) found in S. cerevisiae. The mutant lacking VdSsk2 resulted in equivalent stress-related phenotypes as exhibited by the ΔVdpbs2 or ΔVdhog1 strains. In contrast to the phenotypes of the ΔVdSsk2, ΔVdpbs2, ΔVdhog1, the ΔVdste11 strain did not display any stress-sensitive phenotypes. It is well-known that hypersensitivity to high osmolarity and increased resistance to fludioxonil are two hallmarks of the Hog pathway and also appeared in the deletion mutants of Hog1 cascade components in other filamentous fungi, such as Beauveria bassiana (36), Cochliobolus heterostrophus (37), Botrytis cinerea (15), Mycosphaerella graminicola (17), Colletotrichum lagenarium (38), Cryphonectria parasitica (18), Aspergillus nidulans (39), and Magnaporthe grisea (19). More importantly, biochemical assays have demonstrated that Hog1 phosphorylation was inhibited in the absence of Ssk2, Pbs2, or Hog1, but not altered in the absence of Ste11, in response to high osmolarity (40, 41). This also supports the conclusion that VdSsk2, VdPbs2, and VdHog1 are sequentially phosphorylated in V. dahliae in response to hyperosmolarity, but not VdSte11. Taken together, in contrast to findings in model yeast systems, we propose that the Hog signaling occurs through sequential phosphorylation of the Ssk2, Pbs2, and Hog1 protein orthologs in filamentous fungi. However, it remains to be determined whether single or multiple histone kinases or other kinases function upstream of the cascade in response to various stressor cues. Using yeast two-hybrid assays, we found that neither VdSsk2 nor Ste11 physically interacts with VdPbs2 (data not shown). Exploring the biochemical and genetic connections between sensors, histone kinases, and the Hog pathway will be a future goal.
The budding yeast S. cerevisiae undergoes filamentous growth, which requires MAP kinase pathways (42). This seminal discovery led to knowledge that S. cerevisiae Ste11 is required for filamentous growth, whereas Ssk2/Ssk22 is not (43), which was consistent with our observations that the ΔVdSte11 mutant was defective in filamentous growth while the ΔVdSsk2 mutant had no obvious difference on filamentous growth.
The findings herein clarified distinctive roles of VdSsk2 and VdSte11 in the development of melanized microsclerotia in V. dahliae. Melanized microsclerotial formation is critically important in the life cycle of V. dahliae for both survival and spread (1). Verticillium virulence and the development of microsclerotia increasingly appear to be linked (44), though the basis for this correlation is not understood. Nevertheless, numerous genes have been characterized as having roles in the regulation of both virulence and microsclerotial development (44). Melanized microsclerotia produced by the fungus originate from hyphal swelling and septation, and then fungal cells within these clusters subsequently become dark due to melanin secretion (45, 46). In the present study, we provided several lines of evidence demonstrating distinctive functions of VdSsk2 and VdSte11 in microsclerotial formation and melanin biosynthesis. The ΔVdSsk2 strain exhibited a severe delay in microsclerotial formation and melanization. Although the microsclerotial development of the ΔVdste11 strain was similar to that observed in the wild type, there was clearly a lack of melanin biosynthesis, suggesting that both VdSsk2 and VdSte11 are involved in melanization of the microsclerotia. Importantly, delayed and reduced melanized microsclerotial formation of the ΔVdSsk2 strain mimics the phenotypes observed after deletion of multiple Hog1 MAPK pathway genes, including VdHog1, VdPbs2, VdMsb, and VdSho1 (28–30, 34). Thus, mutation of VdSsk2 or these components of the Hog MAPK pathway delays and reduces melanized microsclerotial formation in V. dahliae, demonstrating that the Hog pathway plays important roles in microsclerotial formation and melanization.
On the other hand, the ΔVdSte11 strain exhibited a lack of melanin, especially during microsclerotial formation, which is reminiscent of the vmk1 mutant (31). We also found that deletion of VdSte7 does affect melanin biosynthesis, but not microsclerotial formation (not published), indicating that Ste11-Ste7-Kss1 functions as the MAPK cascade in V. dahliae. We also previously reported that VdCmr1 is required for melanin production, but not for microsclerotial development (5). In the current study, we demonstrated that expression of VdCmr1 was significantly downregulated in the ΔVdste11 mutant. We further found that expression of most melanogenesis-associated genes, including that of Vayg1, was completely inhibited in the ΔVdste11 strain. Moreover, the transcription factor VdMsn2 was previously proven to be a negative regulator of microsclerotial formation (27). The expression of VdMsn2 was upregulated in the ΔVdste11 mutant, which supports the observation of microsclerotial formation without melanin production. Taken together, these findings indicate that VdSte11 is required for melanin biosynthesis but not for microsclerotial development. However, melanin production was nevertheless impaired in Hog1 MAPK pathway mutants in culture, suggesting that melanogenesis is dependent on Hog1 signaling. It will be of interest to identify the overlapping mechanisms of melanogenesis regulated by different signaling pathways in future studies.
We also found that Ste11 plays a conserved role in pathogenesis in V. dahliae. Most pathogenic fungi possess infection-related MAPKs that are orthologs of the yeast Fus3/Kss1 MAPK, and these MAPKs play important functions in establishment of various infection strategies. Kss1 orthologs are essential for plant infection in many phytopathogens. For example, the M. oryzae PMK1 is crucial for appressorium formation and controls plant cell-to-cell invasion (47, 48). The genes upstream of PMK1, especially MAPKK MST7 and MAPKKK MST11, as well as the adaptor protein Ste50, are essential for differentiation of penetration structures and plant infection (49, 50). M. oryzae strains lacking Ste11 failed to form appressoria and are nonpathogenic. In multiple pathogenic fungi, the deletion or disruption of Ste11 resulted in the loss of virulence, i.e., Colletotrichum orbiculare (51), Cochliobolus heterostrophus (52), and Candida albicans (53). Indeed, the Ste11-Ste7-Kss1 cascade is required for infection in all phytopathogens examined (8, 54). The current studies show that deletion of VdSte11 causes loss of pathogenicity, as the mutant fails to penetrate and develop invasive growth in V. dahliae.
Compared with Magnaporthe oryzae, very little is known about how the Ste11-Ste7-Kss1 cascade governs virulence and vascular colonization of V. dahliae. Disruption of VdKss1 (previously known as Vmk1) resulted in reduced virulence against a variety of host plants (31). Furthermore, transcription factor Ste12 is a downstream target of the Ste11-Ste7-Kss1 cascade (55, 56). In V. dahliae, Vhb1, the Ste12 homolog, is required for virulence by impairing the ability to colonize the xylem (57). In agreement with these observations, the ΔVdste11 strain was avirulent. Significantly, our present study has highlighted the importance of studying this cascade in virulence of the soilborne fungus. Dissecting the specific roles of each of the VdSte11-VdSte7-VdKss1 cascade members in virulence requires further investigation.
This work focused on elucidating roles of VdSsk2 and VdSte11 in microsclerotial formation, stress responses, and pathogenicity. In doing so, we uncovered cross talk between the Hog cascade with other MAPK pathways in V. dahliae. Strikingly, the ΔVdSsk2 mutant phenocopies the ΔVdPbs2 and ΔVdHog1 mutants under various stressors, the ΔVdSte11 mutant had defects in filamentous growth, suggesting that in V. dahliae, there is an osmotic branch (VdSsk2-VdPbs2-VdHog1) and a filamentous branch (VdSte11). We noted that under high concentrations of Ca2+, the strains lacking a functional Hog cascade (VdSsk2, VdPbs2, and VdHog1) were much more sensitive than the ΔVdCrz1 strain. Crz1 is a key regulator of calcium signaling in fungi, and VdCrz1 plays important roles in Ca2+ signaling and microsclerotia development in V. dahliae (32). Interestingly, consistent with the increased sensitivity of the mutants, the expression of VdCrz1 was significantly downregulated in Hog cascade mutants. The results suggest that VdCrz1 function is partly dependent on a functional Hog cascade, where the cascade directly influences VdCrz1 gene expression induced by Ca2+. We speculate that Vdcrz1 is regulated by the Hog cascade and Ca2+ signaling. How VdCrz1 is regulated by the Hog pathway requires further analysis in V. dahliae. On the other hand, we also found that VdSsk2 and VdSte11 can coregulate several downstream target genes related to melanin biosynthesis, including the transcription factor VdCmr1 and the polyketide synthase (VdPKS1) (5). Similarly, in C. heterostrophus, Chk1 and Mps1 share multiple downstream targets, such as the transcription factor CMR1 and melanin biosynthetic genes (37). Taken together, we propose a simple model in Fig. 8 to account for the difference and convergence in the two MAPK pathways during microsclerotial formation, stress response, and pathogenesis in V. dahliae, indicating that both pathways are distinct and converged.
FIG 8.
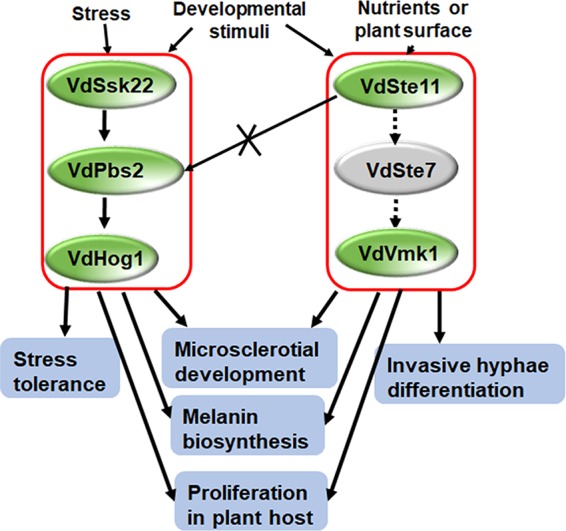
A proposed model for the two MAPK pathways in Verticillium dahliae. Under osmotic stresses, the HOG cascade is activated, leading to an efficient stress response. The HOG cascade is also involved in melanized microsclerotial formation. Our phosphorylation assay demonstrated that a single VdSsk22 activates VdHog1, but not VdSte11, suggesting that the VdSte11 pathway does not transmit phosphorylation signals to the VdPbs2-VdHog1 module. During pathogenesis, the HOG cascade is important for proliferation but not for penetration. However, another MAPK cascade (Ste11-Ste7-Kss1 [Vmk1]) is required for penetration and differentiation of invasive hyphae. This Ste11-Ste7-Kss1 cascade is only required for regulating melanin biosynthesis during melanized microsclerotial formation.
In conclusion, the two MAPKKKs, VdSsk2 and VdSte11, play some distinctive roles in signaling for microsclerotial development, stress responses, and pathogenicity of V. dahliae. Our findings provide compelling evidence indicating that VdSsk2 is necessary for signaling through the VdPbs2-VdHog1 module, while VdSte11 is not necessary for this pathway. Although VdSsk2 (and the cognate Hog cascade) serves a more critical role as a regulator of fungal stress response, and VdSte11 plays a key role in the infection process, cross talk between MAPK cascades converged on microsclerotial development. With this framework, we are pursuing additional questions regarding the roles of MAPK pathways in V. dahliae for development, adaptation to surroundings, and pathogenesis. Characterization of the cross talk between MAPK cascades and other signaling pathways will lead to better understanding of the regulatory network involved in regulation of virulence and formation of microsclerotia as a resting structure of V. dahliae.
MATERIALS AND METHODS
Fungal strains and growth conditions.
The Verticillium dahliae strain XS11 (nondefoliating pathotype, race 2) was isolated from a smoke tree in Fragrant Hills Park, Beijing, China (58) and used as the parental strain to generate gene deletion mutants in this study. All the strain used in this study were primarily incubated on potato dextrose agar medium (PDA) (200 g potato, 20 g glucose, 15 g agar per liter). Conidia used for phenotypic analyses, including growth rate, yield and germination of conidia, and multistress assay, were acquired from a colony that was incubated for 7 days on PDA.
To observe microsclerotial formation, a conidial suspension of V. dahliae (104/ml) was sprayed onto a cellulose membrane (Ø = 80 mm; pore size = 0.22 μm), which was overlaid on solid basal medium (10 g glucose, 0.2 g/liter sodium nitrate, 0.52 g KCl, 0.52 g MgSO4·7H2O, 1.52 g KH2PO4, l μM thiamine HCl, 0.1 μM biotin, 15 g agar per liter). In addition, the conidial suspension was incubated on glass slides that had been smeared with 500 μl solid basal medium (BM) (10 g glucose, 0.2 g sodium nitrate, 0.52 g KCl, 0.52 g MgSO4·7H2O, 1.52 g KH2PO4, 3 μmol thiamine HCl, 0.1 mol biotin, and 15 g agar per liter). Microsclerotial formation was observed and photographed after incubation at 25°C at 48-h intervals. To test sensitivity to multiple stresses, including cell wall inhibitors, such as calcofluor white (CFW) (Sigma-Aldrich) and Congo red (CR) (Sigma-Aldrich), osmotic stresses, and fungicides, conidia of V. dahliae were incubated onto complete medium (CM) (50 ml of 20× nitrate salts, 1 ml of 1,000× trace element, 10 g glucose, 2 g peptone, 1 g yeast extract, 1 g Casamino Acids, 1 ml vitamin solution per liter) described by M. J. Neumann and K. F. Dobinson (59) with or without 0.8 M and 1.0 M NaCl, 1.2 M and 1.4 M sorbitol, 0.4 M and 0.6 M CaCl2, or 10 μg/ml and 30 μg/ml CFW, 25 μg/ml and 75 μg/ml CR at 25°C, respectively. To analyze the chitin distribution in the control and the ΔVdSsk2 and ΔVdSte11 strains, 20 μg/ml CFW was used for staining the hyphae in liquid yeast extract peptone-dextrose broth (YEPD) (3 g yeast extract, 10 g peptone, 20 g dextrose per liter) with or without 0.2 M NaCl for 3 days. Images were captured using a compound microscope (Leica DM 2500).
Immunoblot analyses.
Total proteins were isolated from 48-h fresh mycelia grown in liquid CM cultures, separated on a 12.5% SDS-polyacrylamide gel. Separated total proteins were transferred to a nitrocellulose membrane, and the anti-phospho-p38 monoclonal antibody (P-Hog1; Cell Signaling) was used to detect the phosphorylated form of the V. dahliae Hog1p (VdHog1p). The same blots were stripped and then reprobed with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (catalog no. SRP00849; Saierbio) as the internal control.
Bioinformatic analyses of VdSsk2 and VdSte11 with their homologs.
To identify orthologous genes Ssk2 and Ste11 in V. dahliae, the full-length amino acid sequences of ScSsk22 (YCR073C) and ScSte11p (YLR362W) were used in searches against the V. dahliae genome (60) with blastp (61). The homologs of Ssk2p and Ste11p in other fungi were searched with blastp at the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov). Phylogenetic trees were constructed by MEGA 6.0 (62) with the maximum likelihood algorithm under default settings and 1,000 bootstrap replications. Multiple sequence alignments were performed with ClustalX 2.0 (63).
Deletion and complementation of VdSsk2 and VdSte11 in V. dahliae.
To delete VdSsk2 and VdSte11 in the genome of V. dahliae strain XS11, we used the split-marker method (64). All primers used were included in Table S1 in the supplemental material. The 5′ (1,329-bp) and 3′ (1,380-bp) flanking sequences of VdSsk2 were amplified with primer pairs LY120/LY121 and LY158/LY159, respectively. The 5′ (972-bp) and 3′ (1,078-bp) flanking sequence of VdSte11 were amplified with primer pairs LE9/LE13 and LE7/LE8, respectively. These flanking segments overlapped with Geneticin resistance cassette using primer pair Geneticinfor/Geneticinrev (for stands for forward, and rev stands for reverse). The deletion cassettes of VdSsk2 were obtained with primer pairs LY120/Geneticinrev and Geneticinfor/LY159, and the deletion cassettes of VdSte11 were obtained with primer pair LE9/Geneticinrev and Geneticinfor/LE8. Each of these products was verified by sequencing and then used for protoplast transformation in strain XS11. To obtain the VdSsk2 complemented strain, a segment of DNA (5,951 bp) was amplified using primer pair LY119/LY124, which contain the native promoter and coding sequences of VdSsk2. To obtain the VdSte11 complemented strain, the wild-type coding sequence of VdSte11 was amplified with primer pairs LE9/LE6 (2,872 bp), and a fragment of DNA encoding an external promoter TrpC (464 bp) that was amplified from pRF-HU2 plasmid with primer pairs YJ104/YJ105, and both fragments were included in a final fusion PCR. The fusion products were transformed with the hygromycin resistance gene cassette into protoplasts of the ΔVdSsk2 or ΔVdSte11 deletion mutant by a polyethylene glycol (PEG) method (58). All transformants were verified with the external screening primers LY192/LY193 and the internal screening primers LY190/LY191.
Primers used in the study. Download Table S1, DOCX file, 0.02 MB (18KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
RNA extraction and quantitative real-time PCR.
For detection of the expression levels of genes with known roles in melanin biosynthesis and cell wall biogenesis, the mycelia of XS11, ΔVdSsk2 and ΔVdste11 strains were collected after 7 days of incubation on PDA at 25°C. For calcium treatment, the mycelia of XS11, ΔVdSsk2, ΔVdpbs2, ΔVdhog1, and ΔVdcrz1 strains were incubated into liquid YEPD for 3 days, filtered, washed twice with water, and stimulated with 0.3 M Ca2+ for 30 min for RNA extraction. Total RNA from the mycelia described above was extracted using TRIzol reagent (Invitrogen) and purified with an RNA minikit (Ambion). The cDNA from these strains was synthesized using SuperScript III reverse transcriptase (Invitrogen). Reverse transcription-quantitative PCR (RT-qPCR) was conducted with SYBR green (SuperReal-Premix Plus; Tiangen, China). All of these reactions were run on an ABI7500 real-time PCR system (Applied Biosystems, USA), and the agents for these assays were used in accordance with the manufacturer’s instructions. The cycling conditions for the RT-qPCR experiments included an initial denaturation at 95°C for 15 min, followed by 40 cycles, with 1 cycle consisting of 95°C for 10 s and 60°C for 32 s. The results were calculated by the 2−ΔΔCT method (65). The V. dahliae β-tubulin gene was used as the internal reference for all RT-qPCR analyses by the method of Duressa et al. (7).
Nine genes used for RT-qPCR analyes were listed in Table 1. Four other genes included 1,3-β-glucan synthase (VDAG_02340 and VDAG_02341), glucan synthesis regulatory protein (VDAG_08428), and VdCrz1 (VDAG_03208). All primers used for these analyses are listed in Table S1.
Pathogenicity and penetration assays.
Pathogenicity assays were performed in greenhouses with tobacco seedlings using a root dip method as previously reported by D. Xiong et al. (66). Conidia were filtered from liquid CM after 8 days of incubation and diluted to 2 × 106/ml with distilled water. Three-month-old tobacco seedlings were washed briefly in tap water, soaked in the conidial suspension for 10 min, and replanted into autoclaved soil. The observations for pathogenicity experiments were performed at every 5 days postinoculation (dpi). To determine the ability of strains to approach the vascular system in tobacco, at 5 weeks postinoculation, stem sections around above 10 cm soil were collected and placed on PDA medium. PDA plates were incubated at 25°C for 7 days for observing V. dahliae colonies. The fungal colonies were then transferred to fresh PDA for another 10 days for imaging. The experiment was repeated three times with four replicates.
To observe the ability of the V. dahliae strains to penetrate onion epidermis membrane and cellophane membrane, conidiospores of VdSsk2 and VdSte11 deletion strains were collected from PDA after 7 days of incubation, diluted to 104/ml with sterile water, and inoculated onto each surface at 25°C. Observations of hyphopodia and penetration pegs were performed, and images were captured using a compound microscope (Leica DM 2500) at 24-h intervals.
ACKNOWLEDGMENTS
The research was supported by Key Project of National Key Research and Development Plan (2018YFD0600200) and the National Natural Science Foundation of China (31570636).
Y. Wang conceived the experiments. J. Yu, T. Li, L. Tian, and C. Tang performed the experiments. J. Yu, L. Tian, Y. Wang, and C. Tian analyzed the data. Y. Wang and S. J. Klosterman wrote the manuscript. All authors read and approved the manuscript.
REFERENCES
- 1.Klosterman SJ, Atallah ZK, Vallad GE, Subbarao KV. 2009. Diversity, pathogenicity, and management of Verticillium species. Annu Rev Phytopathol 47:39–62. doi: 10.1146/annurev-phyto-080508-081748. [DOI] [PubMed] [Google Scholar]
- 2.Vallad GE, Subbarao KV. 2008. Colonization of resistant and susceptible lettuce cultivars by a green fluorescent protein-tagged isolate of Verticillium dahliae. Phytopathology 98:871–885. doi: 10.1094/PHYTO-98-8-0871. [DOI] [PubMed] [Google Scholar]
- 3.Klimes A, Dobinson KF. 2006. A hydrophobin gene, VDH1, is involved in microsclerotial development and spore viability in the plant pathogen Verticillium dahliae. Fungal Genet Biol 43:283–294. doi: 10.1016/j.fgb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Xiong D, Wang Y, Ma J, Klosterman SJ, Xiao S, Tian C. 2014. Deep mRNA sequencing reveals stage-specific transcriptome alterations during microsclerotia development in the smoke tree vascular wilt pathogen, Verticillium dahliae. BMC Genomics 15:324. doi: 10.1186/1471-2164-15-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Hu X, Fang Y, Anchieta A, Goldman PH, Hernandez G, Klosterman SJ. 2018. Transcription factor VdCmr1 is required for pigment production, protection from UV irradiation, and regulates expression of melanin biosynthetic genes in Verticillium dahliae. Microbiology 164:685–696. doi: 10.1099/mic.0.000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell AA, Wheeler MH. 1986. Biosynthesis and functions of fungal melanins. Annu Rev Phytopathol 24:411–451. doi: 10.1146/annurev.py.24.090186.002211. [DOI] [Google Scholar]
- 7.Duressa D, Anchieta A, Chen D, Klimes A, Garcia-Pedrajas MD, Dobinson KF, Klosterman SJ. 2013. RNA-seq analyses of gene expression in the microsclerotia of Verticillium dahliae. BMC Genomics 14:607. doi: 10.1186/1471-2164-14-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turra D, Segorbe D, Di Pietro A. 2014. Protein kinases in plant-pathogenic fungi: conserved regulators of infection. Annu Rev Phytopathol 52:267–288. doi: 10.1146/annurev-phyto-102313-050143. [DOI] [PubMed] [Google Scholar]
- 9.Hamel LP, Nicole MC, Duplessis S, Ellis BE. 2012. Mitogen-activated protein kinase signaling in plant-interacting fungi: distinct messages from conserved messengers. Plant Cell 24:1327–1351. doi: 10.1105/tpc.112.096156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banuett F. 1998. Signalling in the yeasts: an informational cascade with links to the filamentous fungi. Microbiol Mol Biol Rev 62:249–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda T, Takekawa M, Saito H. 1995. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science 269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- 12.Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86:865–875. doi: 10.1016/S0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 13.Gustin MC, Albertyn J, Alexander M, Davenport K. 1998. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev 62:1264–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li A, Wang Y, Tao K, Dong S, Huang Q, Dai T, Zheng X, Wang Y. 2010. PsSAK1, a stress-activated MAP kinase of Phytophthora sojae, is required for zoospore viability and infection of soybean. Mol Plant Microbe Interact 23:1022–1031. doi: 10.1094/MPMI-23-8-1022. [DOI] [PubMed] [Google Scholar]
- 15.Segmuller N, Ellendorf U, Tudzynski B, Tudzynski P. 2007. BcSAK1, a stress-activated mitogen-activated protein kinase, is involved in vegetative differentiation and pathogenicity in Botrytis cinerea. Eukaryot Cell 6:211–221. doi: 10.1128/EC.00153-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriwaki A, Kubo E, Arase S, Kihara J. 2006. Disruption of SRM1, a mitogen-activated protein kinase gene, affects sensitivity to osmotic and ultraviolet stressors in the phytopathogenic fungus Bipolaris oryzae. FEMS Microbiol Lett 257:253–261. doi: 10.1111/j.1574-6968.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 17.Mehrabi R, Zwiers LH, de Waard MA, Kema GH. 2006. MgHog1 regulates dimorphism and pathogenicity in the fungal wheat pathogen Mycosphaerella graminicola. Mol Plant Microbe Interact 19:1262–1269. doi: 10.1094/MPMI-19-1262. [DOI] [PubMed] [Google Scholar]
- 18.Park SM, Choi ES, Kim MJ, Cha BJ, Yang MS, Kim DH. 2004. Characterization of HOG1 homologue, CpMK1, from Cryphonectria parasitica and evidence for hypovirus-mediated perturbation of its phosphorylation in response to hypertonic stress. Mol Microbiol 51:1267–1277. doi: 10.1111/j.1365-2958.2004.03919.x. [DOI] [PubMed] [Google Scholar]
- 19.Dixon KP, Xu JR, Smirnoff N, Talbot NJ. 1999. Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium-mediated plant infection by Magnaporthe grisea. Plant Cell 11:2045–2058. doi: 10.1105/tpc.11.10.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Zhang S, Hou R, Zhao Z, Zheng Q, Xu Q, Zheng D, Wang G, Liu H, Gao X, Ma JW, Kistler HC, Kang Z, Xu JR. 2011. Functional analysis of the kinome of the wheat scab fungus Fusarium graminearum. PLoS Pathog 7:e1002460. doi: 10.1371/journal.ppat.1002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berridge MJ, Bootman MD, Roderick HL. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 22.Chin D, Means AR. 2000. Calmodulin: a prototypical calcium sensor. Trends Cell Biol 10:322–328. doi: 10.1016/S0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 23.Stathopoulos AM, Cyert MS. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev 11:3432–3444. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi J, Kim Y, Kim S, Park J, Lee YH. 2009. MoCRZ1, a gene encoding a calcineurin-responsive transcription factor, regulates fungal growth and pathogenicity of Magnaporthe oryzae. Fungal Genet Biol 46:243–254. doi: 10.1016/j.fgb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Zhao Q, Liu K, Zhang Z, Wang Y, Zheng X. 2009. MgCRZ1, a transcription factor of Magnaporthe grisea, controls growth, development and is involved in full virulence. FEMS Microbiol Lett 293:160–169. doi: 10.1111/j.1574-6968.2009.01524.x. [DOI] [PubMed] [Google Scholar]
- 26.Schumacher J, de Larrinoa IF, Tudzynski B. 2008. Calcineurin-responsive zinc finger transcription factor CRZ1 of Botrytis cinerea is required for growth, development, and full virulence on bean plants. Eukaryot Cell 7:584–601. doi: 10.1128/EC.00426-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian L, Yu J, Wang Y, Tian C. 2017. The C2H2 transcription factor VdMsn2 controls hyphal growth, microsclerotia formation, and virulence of Verticillium dahliae. Fungal Biol 121:1001–1010. doi: 10.1016/j.funbio.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Tian L, Xiong D, Klosterman SJ, Xiao S, Tian C. 2016. The mitogen-activated protein kinase gene, VdHog1, regulates osmotic stress response, microsclerotia formation and virulence in Verticillium dahliae. Fungal Genet Biol 88:13–23. doi: 10.1016/j.fgb.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Tian L, Wang Y, Yu J, Xiong D, Zhao H, Tian C. 2016. The mitogen-activated protein kinase kinase VdPbs2 of Verticillium dahliae regulates microsclerotia formation, stress response, and plant infection. Front Microbiol 7:1532. doi: 10.3389/fmicb.2016.01532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi XY, Zhou S, Shang XG, Wang XY. 2016. VdSho1 regulates growth, oxidant adaptation and virulence in Verticillium dahliae. J Phytopathol 164:1064–1074. doi: 10.1111/jph.12527. [DOI] [Google Scholar]
- 31.Rauyaree P, Ospina-Giraldo MD, Kang S, Bhat RG, Subbarao KV, Grant SJ, Dobinson KF. 2005. Mutations in VMK1, a mitogen-activated protein kinase gene, affect microsclerotia formation and pathogenicity in Verticillium dahliae. Curr Genet 48:109–116. doi: 10.1007/s00294-005-0586-0. [DOI] [PubMed] [Google Scholar]
- 32.Xiong D, Wang Y, Tang C, Fang Y, Zou J, Tian C. 2015. VdCrz1 is involved in microsclerotia formation and required for full virulence in Verticillium dahliae. Fungal Genet Biol 82:201–212. doi: 10.1016/j.fgb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Madhani HD, Styles CA, Fink GR. 1997. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell 91:673–684. doi: 10.1016/S0092-8674(00)80454-7. [DOI] [PubMed] [Google Scholar]
- 34.Tian L, Xu J, Zhou L, Guo W. 2014. VdMsb regulates virulence and microsclerotia production in the fungal plant pathogen Verticillium dahliae. Gene 550:238–244. doi: 10.1016/j.gene.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 35.Ha YS, Covert SF, Momany M. 2006. FsFKS1, the 1,3-beta-glucan synthase from the caspofungin-resistant fungus Fusarium solani. Eukaryot Cell 5:1036–1042. doi: 10.1128/EC.00030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Wang ZK, Sun HH, Ying SH, Feng MG. 2017. Characterization of the Hog1 MAPK pathway in the entomopathogenic fungus Beauveria bassiana. Environ Microbiol 19:1808–1821. doi: 10.1111/1462-2920.13671. [DOI] [PubMed] [Google Scholar]
- 37.Igbaria A, Lev S, Rose MS, Lee BN, Hadar R, Degani O, Horwitz BA. 2008. Distinct and combined roles of the MAP kinases of Cochliobolus heterostrophus in virulence and stress responses. Mol Plant Microbe Interact 21:769–780. doi: 10.1094/MPMI-21-6-0769. [DOI] [PubMed] [Google Scholar]
- 38.Kojima K, Takano Y, Yoshimi A, Tanaka C, Kikuchi T, Okuno T. 2004. Fungicide activity through activation of a fungal signalling pathway. Mol Microbiol 53:1785–1796. doi: 10.1111/j.1365-2958.2004.04244.x. [DOI] [PubMed] [Google Scholar]
- 39.Kawasaki L, Sanchez O, Shiozaki K, Aguirre J. 2002. SakA MAP kinase is involved in stress signal transduction, sexual development and spore viability in Aspergillus nidulans. Mol Microbiol 45:1153–1163. doi: 10.1046/j.1365-2958.2002.03087.x. [DOI] [PubMed] [Google Scholar]
- 40.Noguchi R, Banno S, Ichikawa R, Fukumori F, Ichiishi A, Kimura M, Yamaguchi I, Fujimura M. 2007. Identification of OS-2 MAP kinase-dependent genes induced in response to osmotic stress, antifungal agent fludioxonil, and heat shock in Neurospora crassa. Fungal Genet Biol 44:208–218. doi: 10.1016/j.fgb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Lee YM, Kim E, An J, Lee Y, Choi E, Choi W, Moon E, Kim W. 2017. Dissection of the HOG pathway activated by hydrogen peroxide in Saccharomyces cerevisiae. Environ Microbiol 19:584–597. doi: 10.1111/1462-2920.13499. [DOI] [PubMed] [Google Scholar]
- 42.Gimeno CJ, Ljungdahl PO, Styles CA, Fink GR. 1992. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077–1090. doi: 10.1016/0092-8674(92)90079-R. [DOI] [PubMed] [Google Scholar]
- 43.Roberts RL, Fink GR. 1994. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev 8:2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- 44.Klimes A, Dobinson KF, Thomma BP, Klosterman SJ. 2015. Genomics spurs rapid advances in our understanding of the biology of vascular wilt pathogens in the genus Verticillium. Annu Rev Phytopathol 53:181–198. doi: 10.1146/annurev-phyto-080614-120224. [DOI] [PubMed] [Google Scholar]
- 45.Griffiths DA. 1970. The fine structure of developing microsclerotia of Verticillium dahliae Kleb. Archiv Mikrobiol 74:207–212. doi: 10.1007/BF00408881. [DOI] [Google Scholar]
- 46.Coley-Smith JR, Cooke RC. 1971. Survival and germination of fungal sclerotia. Annu Rev Phytopathol 9:65–92. doi: 10.1146/annurev.py.09.090171.000433. [DOI] [Google Scholar]
- 47.Sakulkoo W, Oses-Ruiz M, Oliveira Garcia E, Soanes DM, Littlejohn GR, Hacker C, Correia A, Valent B, Talbot NJ. 2018. A single fungal MAP kinase controls plant cell-to-cell invasion by the rice blast fungus. Science 359:1399–1403. doi: 10.1126/science.aaq0892. [DOI] [PubMed] [Google Scholar]
- 48.Xu JR, Hamer JE. 1996. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev 10:2696–2706. doi: 10.1101/gad.10.21.2696. [DOI] [PubMed] [Google Scholar]
- 49.Zhao X, Kim Y, Park G, Xu J. 2005. A mitogen-activated protein kinase cascade regulating infection-related morphogenesis in Magnaporthe grisea. Plant Cell 17:1317–1329. doi: 10.1105/tpc.104.029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park G, Xue C, Zhao X, Kim Y, Orbach M, Xu JR. 2006. Multiple upstream signals converge on the adaptor protein Mst50 in Magnaporthe grisea. Plant Cell 18:2822–2835. doi: 10.1105/tpc.105.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sakaguchi A, Tsuji G, Kubo Y. 2010. A yeast STE11 homologue CoMEKK1 is essential for pathogenesis-related morphogenesis in Colletotrichum orbiculare. Mol Plant Microbe Interact 23:1563–1572. doi: 10.1094/MPMI-03-10-0051. [DOI] [PubMed] [Google Scholar]
- 52.Izumitsu K, Yoshimi A, Kubo D, Morita A, Saitoh Y, Tanaka C. 2009. The MAPKK kinase ChSte11 regulates sexual/asexual development, melanization, pathogenicity, and adaptation to oxidative stress in Cochliobolus heterostrophus. Curr Genet 55:439–448. doi: 10.1007/s00294-009-0257-7. [DOI] [PubMed] [Google Scholar]
- 53.Csank C, Schroppel K, Leberer E, Harcus D, Mohamed O, Meloche S, Thomas DY, Whiteway M. 1998. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect Immun 66:2713–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang C, Zhang X, Liu H, Xu JR. 2018. Mitogen-activated protein kinase signaling in plant pathogenic fungi. PLoS Pathog 14:e1006875. doi: 10.1371/journal.ppat.1006875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rispail N, Di Pietro A. 2010. The homeodomain transcription factor Ste12: connecting fungal MAPK signalling to plant pathogenicity. Commun Integr Biol 3:327–332. doi: 10.4161/cib.3.4.11908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elion EA, Qi M, Chen W. 2005. Signaling specificity in yeast. Science 307:687–688. doi: 10.1126/science.1109500. [DOI] [PubMed] [Google Scholar]
- 57.Sarmiento-Villamil JL, Prieto P, Klosterman SJ, García-Pedrajas MD. 2018. Characterization of two homeodomain transcription factors with critical but distinct roles in virulence in the vascular pathogen Verticillium dahliae. Mol Plant Pathol 19:986–1004. doi: 10.1111/mpp.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Xiao S, Xiong D, Tian C. 2013. Genetic transformation, infection process and qPCR quantification of Verticillium dahliae on smoke-tree Cotinus coggygria. Australas Plant Pathol 42:33–41. doi: 10.1007/s13313-012-0172-0. [DOI] [Google Scholar]
- 59.Neumann MJ, Dobinson KF. 2003. Sequence tag analysis of gene expression during pathogenic growth and microsclerotia development in the vascular wilt pathogen Verticillium dahliae. Fungal Genet Biol 38:54–62. doi: 10.1016/S1087-1845(02)00507-8. [DOI] [PubMed] [Google Scholar]
- 60.Klosterman SJ, Subbarao KV, Kang SC, Veronese P, Gold SE, Thomma B, Chen ZH, Henrissat B, Lee YH, Park J, Garcia-Pedrajas MD, Barbara DJ, Anchieta A, de Jonge R, Santhanam P, Maruthachalam K, Atallah Z, Amyotte SG, Paz Z, Inderbitzin P, Hayes RJ, Heiman DI, Young S, Zeng QD, Engels R, Galagan J, Cuomo CA, Dobinson KF, Ma LJ. 2011. Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathog 7:e1002137. doi: 10.1371/journal.ppat.1002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 62.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 64.Goswami RS. 2012. Targeted gene replacement in fungi using a split-marker approach. Methods Mol Biol 835:255–269. doi: 10.1007/978-1-61779-501-5_16. [DOI] [PubMed] [Google Scholar]
- 65.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 66.Xiong D, Wang Y, Tian L, Tian C. 2016. MADS-box transcription factor VdMcm1 regulates conidiation, microsclerotia formation, pathogenicity, and secondary metabolism of Verticillium dahliae. Front Microbiol 7:1192. doi: 10.3389/fmicb.2016.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan R, Klosterman SJ, Wang C, Subbarao KV, Xu X, Shang W, Hu X. 2017. Vayg1 is required for microsclerotium formation and melanin production in Verticillium dahliae. Fungal Genet Biol 98:1–11. doi: 10.1016/j.fgb.2016.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence analyses of VdSsk2 from Verticillium dahliae and its orthologs. (a) Schematic charts of putative conserved domains encoded by VdSsk2 compared with S. cerevisiae Ssk2p. STKc_MEKK4 domains are shown in boxes. (b) Unrooted neighbor-joining tree of VdSsk2 protein was constructed with its orthologs from other fungi. The phylogenetic tree was constructed using MEGA 6.0 (62) using the full-length protein sequences. Bootstrap values (as a percentage) obtained with 1,000 replicates are indicated on branches, and branch lengths correspond to genetic distance (the scale bar at the lower left corresponds to a genetic distance of 0.1). The amino acid sequences include VDAG_08787 and its homologs from Saccharomyces cerevisiae (ScSsk22, YCR073C; ScSSK2, YNR031C), Verticillium alfalfae (VDBG_04429), Metarhizium robertsii (MAA_04290), Neurospora crassa (NCU03071), Fusarium oxysporum (FOXG_00643), Magnaporthe oryzae (MGG_00183), Botrytis cinerea (BC1G_04606), Ustilago maydis (UMAG_01544), Cryptococcus neoformans (CNL05560), Aspergillus niger (ANI_1_450074), Candida albicans (CAALFM_C405030CA), Beauveria bassiana (EJP70068), and Metarhizium robertsii (XP_007820479). Download FIG S1, TIF file, 2.4 MB (2.5MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Sequence analyses of VdSte11 from Verticillium dahliae and its orthologs. (a) Schematic charts of conserved protein domains encoded by VdSte11 compared with S. cerevisiae Ste11p. Domains, including SAM, Ras_bdg_2, and PKc_like superfamily are shown in boxes of different colors. (b) Unrooted neighbor-joining tree of VdSte11 protein was constructed with its orthologs from other fungi. A phylogenetic tree was constructed using MEGA 6.0 (62) using the full-length protein sequences. Bootstrap values (as a percentage) obtained with 1,000 replicates are indicated on branches, and branch lengths correspond to genetic distance (a scale bar at the lower left corresponds to a genetic distance of 0.1). The amino acid sequences include VDAG_05822 and its homologs from S. cerevisiae (YLR362W), V. alfalfae (VDBG_06755), F. oxysporum (FOXG_09411), M. oryzae (MGG_14847), N. crassa (NCU06182), M. robertsii (MAA_03529), B. bassiana (EJP69245.1), B. cinerea (ACJ06645.1), A. niger (XP_001398357.2), A. nidulans (AN2269.2), and C. albicans (C203770CA). Download FIG S2, TIF file, 2.4 MB (2.5MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Deletion of VdSsk2 and VdSte11 in Verticillium dahliae. (a) Schematic diagram of construction of gene deletion cassettes using the split marker method (64). (b) Confirmation of the VdSte11 gene deletion by PCR. Following PCR amplification with the internal screening primer pairs (RT-primer) LE13/LE14 and the specific primer pairs LE9/LE14, a 450-bp band and a 1,943-bp band were detected for the wild-type strain and the complementary strains of VdSte11 gene, but no band showed in the transformant e16. A 1,700-bp band was amplified with the universal primer M13F/M13R from the genomic DNA of the transformant, e16. (c) Confirmation of the VdSsk2 gene deletion mutant by PCR. Using the internal screening primer pairs, LY190/LY191, a 318-bp band was amplified from genomic DNA from the wild-type strain XS11 and the complemented strains of VdSsk2 gene, but no band was found in ΔVdSsk2-2 ΔVdSsk2-50 strains. (d) The internal screening primer pairs LE13/LE14 and LY190/LY191 were used to confirm VdSte11 and VdSsk2 gene complementation with cDNA by reverse transcription-PCR, respectively. Primer pairs VdBt-up/VdBt-down were used to verify the quality of the genomic DNA and cDNA of all strains in this assay. Download FIG S3, TIF file, 1.8 MB (1.9MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Colony morphology in the wild-type (XS11), ΔVdSte11, and ΔVdSsk2 strains. Colony morphology of XS11, ΔVdSsk2, and ΔVdSte11 strains, and the respective complemented strains were incubated on CM and PDA for 7 days. Bar indicates 1 cm. Download FIG S4, TIF file, 2.3 MB (2.3MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Microsclerotial formation in the wild type (WT), ΔVdSte11, and ΔVdSsk2 strains at 60 dpi. Melanized microsclerotial formation of the strains was observed on the cellulose membranes incubated at 25°C for 60 days. Microscopy was used to assess microsclerotial formation. Download FIG S5, TIF file, 2.5 MB (2.6MB, tif) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Primers used in the study. Download Table S1, DOCX file, 0.02 MB (18KB, docx) .
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.