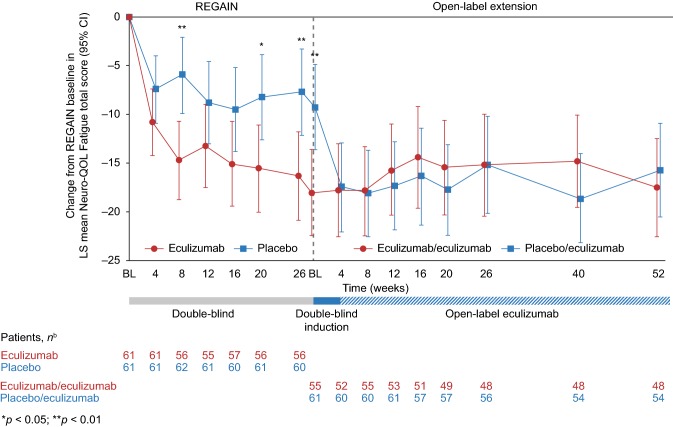
Fig. 1.
Change in Neuro-QOL Fatigue subscale total score from REGAIN baseline to week 52 of the open-label study using a repeated-measures model.aaA repeated-measures model using the restricted maximum likelihood for the changes from baseline was used to compare the two treatment groups at each assessment visit and over time. The model included the following terms: treatment, visit, treatment by visit interaction, pooled Myasthenia Gravis Foundation of America (MGFA) randomization stratification variable (based on their MGFA classification at screening, patients were assigned to one of two categories: IIa/IIIa/IVa [a, symptoms predominantly affecting limb or axial muscles, or both] or IIb/IIIb/IVb [b, symptoms predominantly affecting oropharyngeal or respiratory muscles, or both]), and Neuro-QOL Fatigue total score at baseline. bNumber of patients who completed the assessment at each time point. Some patients did not complete all items of the questionnaire at every timepoint. When this occurred, total scores could not be computed at that time point. Missing scores were not imputed. BL baseline, CI confidence interval, Neuro-QOL Fatigue Quality of Life in Neurological Disorders Fatigue subscale, REGAIN Eculizumab for REfractory GenerAlIzed MyastheNia Gravis