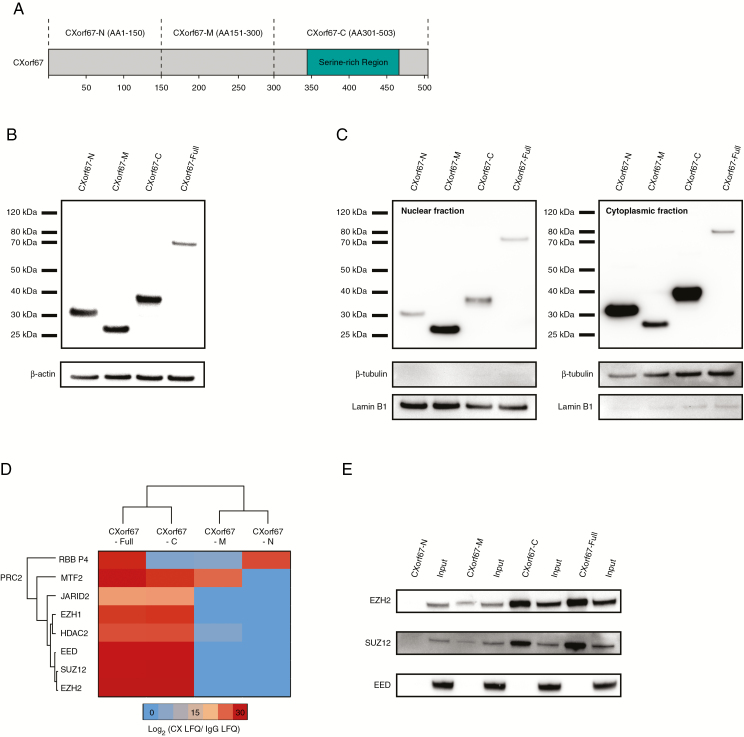
Fig. 1.
Mass spectrometry analysis of CXorf67 truncates reveals interaction of the C-terminus of CXorf67 with PRC2 core components. (A) Overview of CXorf67 and the 3 truncates used in this study. The serine-rich region of the protein is highlighted in turquoise. (B) Detection of FLAG-tagged CXorf67 truncates and the full-length protein in lysates of HEK293T cells after transduction. (C) Western blot analysis of nuclear and cytoplasmic fractions obtained from transduced HEK293T cells. Both fractions contain CXorf67 truncates and the full-length protein. Lamin B1 was used as a nuclear loading control and β-tubulin as a loading control for the cytoplasmic fraction. (D) Heatmap depicting PRC2 components interacting with CXorf67-Full and at least 1 truncate. The scale bar depicts the ratio of the log2 signal intensities (LFQ) of PRC2 related proteins within each CXorf67 variant experiment over their respective immunoglobulin G control. (E) Validation of CXorf67-C interaction with EZH2 and SUZ12 by co-IP. A faint interaction can also be observed for CXorf67-M.