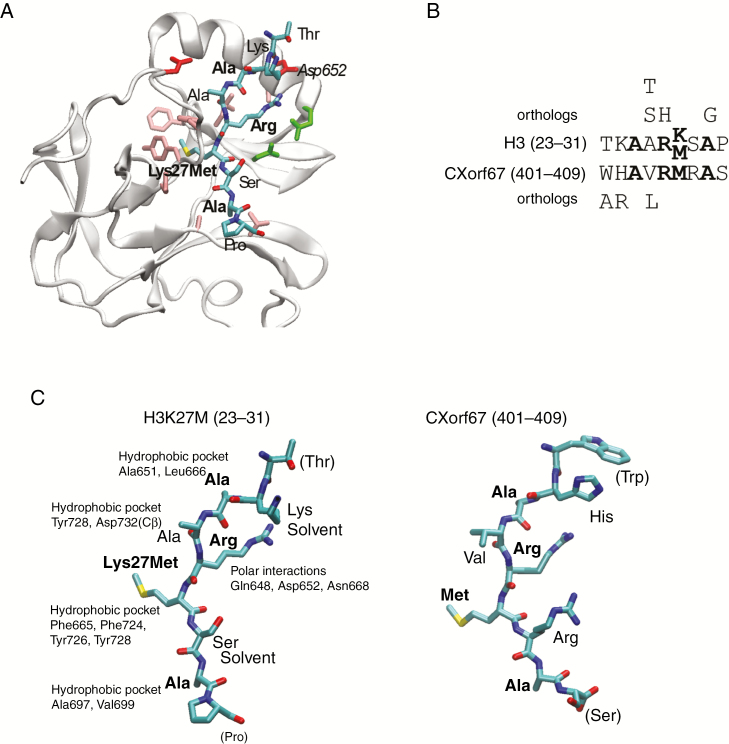
Fig. 4.
CXorf67 inhibits PRC2 via a small, highly conserved peptide sequence located in its C-terminus. (A) Structure of EZH2 bound to H3K27M22 where the bound peptide is shown as sticks and colored according to atom type. Residues in EZH2 binding to the peptide are shown as sticks and colored according to residue type: hydrophobic, pink; polar, green; negative, red. (B) Alignment of the peptide regions from H3 and CXorf67, where conserved residues are in bold. Residues above and below the alignment are those identified as allowed substitutions by perusing all orthologs of the 2 proteins in eggNOG.27 (C) The 2 peptides in the same orientation, where part of the CXorf67 peptide was modeled into the EZH2 structure using Modeller.23 The relevant binding pocket residues in EZH2 are labeled for each residue in H3K27M and conserved residues are shown in boldface. Structures were created using visual molecular dynamics.44