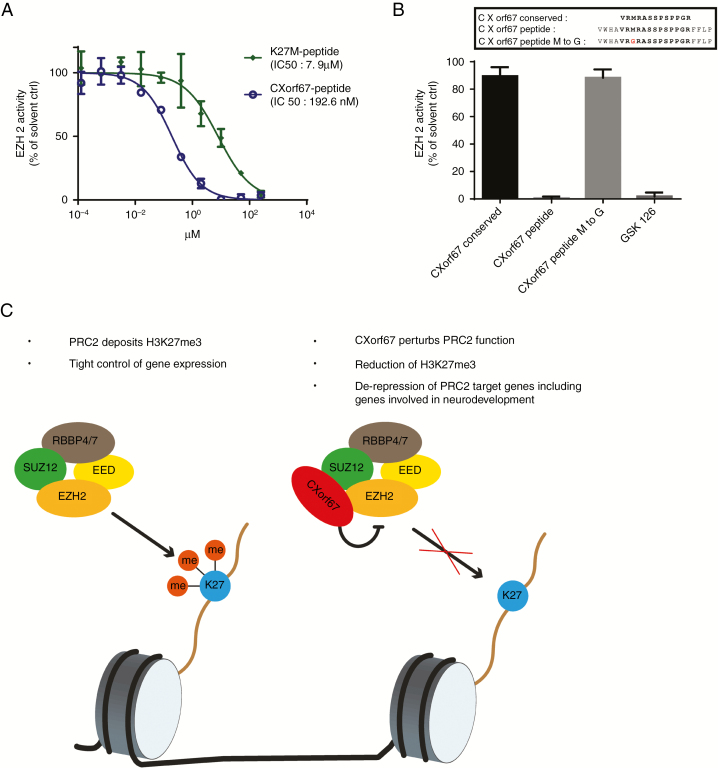
Fig. 5.
A CXorf67 peptide incorporating the conserved region potently inhibits EZH2. (A) Methyltransferase assay to evaluate the inhibitory capabilities of the CXorf67 and the K27M peptides. (B) Evaluation of EZH2 inhibition by CXorf67 peptide variants at a single concentration of 10 µM. Peptide variants encompass the conserved region alone (CXorf67 conserved), the longer variant incorporating surrounding amino acids (CXorf67 peptide) and the longer peptide variant in which the methionine has been replaced by a glycine (CXorf67 peptide M to G). GSK126 was used as a positive control. (C) Proposed model of CXorf67-mediated H3K27 hypomethylation. When CXorf67 is absent, PRC2 mediates trimethylation of H3K27, resulting in a tight regulation of gene expression. In contrast, the presence of CXorf67 results in the inhibition of PRC2. In detail, the conserved domain of CXorf67 sequesters EZH2, thereby abolishing its methyltransferase activity. The resulting decrease in H3K27me3 levels allows the de-repression of PRC2 target genes, including genes involved in neurodevelopment which contributes to the tumorigenesis of PFA ependymoma.