Abstract
The discovery in 2009 that 2′,3′-cAMP exists in biological systems was rapidly followed by identification of 2′,3′-cGMP in cell and tissue extracts. To determine whether 2′,3′-cGMP exists in mammals under physiological conditions, we used ultraperformance LC-MS/MS to measure 2′,3′-cAMP and 2′,3′-cGMP in timed urine collections (via direct bladder cannulation) from 25 anesthetized mice. Urinary excretion rates (means ± SE) of 2′,3′-cAMP (15.5 ± 1.8 ng/30 min) and 2′,3′-cGMP (17.9 ± 1.9 ng/30 min) were similar. Mice also excreted 2′-AMP (3.6 ± 1.1 ng/20 min) and 3′-AMP (9.5 ± 1.2 ng/min), hydrolysis products of 2′,3′-cAMP, and 2′-GMP (4.7 ± 1.7 ng/30 min) and 3′-GMP (12.5 ± 1.8 ng/30 min), hydrolysis products of 2′,3′-cGMP. To validate that the chromatographic signals were from these endogenous noncanonical nucleotides, we repeated these experiments in mice (n = 18) lacking 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase), an enzyme known to convert 2′,3′-cyclic nucleotides to their corresponding 2′-nucleotides. In CNPase-knockout mice, urinary excretions of 2′,3′-cAMP, 3′-AMP, 2′,3′-cGMP, and 3′-GMP were increased, while urinary excretions of 2′-AMP and 2′-GMP were decreased. Infusions of exogenous 2′,3′-cAMP increased urinary excretion of 2′,3′-cAMP, 2′-AMP, 3′-AMP, and adenosine, whereas infusions of exogenous 2′,3′-cGMP increased excretion of 2′,3′-cGMP, 2′-GMP, 3′-GMP, and guanosine. Together, these data suggest the endogenous existence of not only a 2′,3′-cAMP-adenosine pathway (2′,3′-cAMP → 2′-AMP/3′-AMP → adenosine), which was previously identified, but also a 2′,3′-cGMP-guanosine pathway (2′,3′-cGMP → 2′-GMP/3′-GMP → guanosine), observed here for the first time. Because it is well known that adenosine and guanosine protect tissues from injury, our data support the concept that both pathways may work together to protect tissues from injury.
Keywords: 2′,3′-cAMP-adenosine pathway; 2′,3′-cGMP; 2′-GMP; 3′-GMP; 2′,3′-cGMP-guanosine pathway
INTRODUCTION
While measuring adenosine 3′,5′-cyclic monophosphate (3′,5′-cAMP) in the renal venous outflow from isolated, perfused rat kidneys, Ren et al. (49) made the serendipitous discovery that adenosine 2′,3′-cyclic monophosphate (2′,3′-cAMP) is also made by and released from the kidney. This was the first unequivocal identification of a nucleoside 2′,3′-cyclic monophosphate (2′,3′-cNMP) in a biological system. In subsequent experiments, Jackson et al. (30) showed that 1) in the in vitro rat kidney, energy depletion stimulates the release of endogenous 2′,3′-cAMP, 2′-AMP, 3′-AMP, and adenosine and 2) kidneys in vitro and in vivo metabolize exogenous 2′,3′-cAMP to 2′-AMP and 3′-AMP and convert exogenous 2′-AMP and 3′-AMP to adenosine. This biochemical pathway (2′,3′-cAMP → 2′-AMP/3′-AMP → adenosine) is known as the 2′,3′-cAMP-adenosine pathway (for reviews see Refs. 15 and 16). Verrier et al. (58) confirmed the in vivo existence in mice and humans of an endogenous 2′,3′-cAMP-adenosine pathway.
After the identification of 2′,3′-cAMP in the rat kidney, there were several reports of 2′,3′-cGMP in extracts of biological samples, including mammalian organs (6, 32, 56), human embryonic kidney (HEK-293T) and HuT-78 cells (3), plant tissue (39, 55), bacteria (11, 43), and algae (4). However, it is unknown whether 2′,3′-cGMP exists under physiological conditions and whether there is a corresponding 2′,3′-cGMP-guanosine pathway (2′,3′-cGMP → 2′-GMP/3′-GMP → guanosine). To determine whether 2′,3′-cGMP and 2′,3′-cGMP-guanosine pathways exist in vivo under physiological conditions, we obtained timed urine collections from mice with carefully placed bladder catheters. Then, using ultraperformance LC-MS/MS (UPLC-MS/MS), we analyzed urine without extraction for 2′,3′-cAMP, 2′,3-cGMP, 2′-AMP, 2′-GMP, 3′-AMP, 3′-GMP, adenosine, and guanosine. To confirm that the signals (i.e., mass transitions) used to identify 2′,3′-cGMP, 2′-GMP, and 3′-GMP were indeed detecting these compounds, we also performed these experiments in mice lacking 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase), an enzyme known to convert 2′,3′-cyclic nucleotides to their corresponding 2′-nucleotides. In addition, we examined the effects of infusions of exogenous 2′,3′-cGMP on urinary excretion of 2′,3′-cGMP, 2′-GMP, 3′-GMP, and guanosine.
MATERIALS AND METHODS
Animals.
Male and female homozygous CNPase-knockout (CNPase−/−) and wild-type (CNPase+/+) mice were bred and genotyped at the University of Pittsburgh, as previously described (58). Mice were ~20 wk of age at the time of the study, and the females were sexually mature, with intact ovaries. The University of Pittsburgh Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, Revised 1996).
Chemicals.
2′,3′-cAMP, 2′,3-cGMP, 2′-AMP, 2′-GMP, 3′-AMP, 3′-GMP, adenosine, and guanosine were obtained from Sigma-Aldrich (St. Louis, MO). Heavy-isotope internal standards were obtained from the following sources: 2′,3′-[13C5]cAMP and 3′,5′-[13C5]cGMP from Toronto Research Chemicals (Toronto, ON, Canada), 2′-[13C5]AMP and 3′-[13C5]AMP from 13C Molecular (Fayetteville, NC), and 5′-[13C5]GMP, [13C10]adenosine, and [13C10,15N5]guanosine from Medical Isotopes (Pelham, NH).
Animal experiments.
CNPase+/+ or CNPase−/− mice were anesthetized with thiobutabarbital (Inactin; 100 mg/kg ip) and placed on a heating system to maintain body temperature at 37°C. To support a patent airway, a PE-90 cannula was inserted into the trachea. Next, a PE-10 catheter was inserted into the carotid artery and attached to a pressure transducer (Micro-Med, Louisville, KY) that was connected to a blood pressure analyzer (Micro-Med). Mean arterial blood pressure (MABP) was recorded continuously using the PowerLab data acquisition system and LabChart software (ADInstruments, Colorado Springs, CO). For intravenous infusions, a PE-10 catheter was inserted into the jugular vein, and an infusion of 2.45% albumin in 0.9% saline was initiated at 10 μl/min to maintain hemodynamic stability and ensure a robust urine flow.
For timed urine collections, we employed a method we developed for cannulating the mouse bladder that provides unimpeded flow of urine into the collection device (24). A segment of PE-50 tubing was inserted into silicone tubing, and the silicone-covered PE-50 tubing was placed in the bladder via a small hole that was cut in the rostral end of the bladder using a cautery. The bladder catheter was advanced toward the urethra and secured in place with a 4-0 suture ligated around the body of the bladder at a level rostral to the entry location of the ureters into the bladder. The PE-50 tubing provided a rigid body for securing the catheter in place, and the outer silicone tubing protected the bladder from injury and allowed more flexibility in the catheter exiting the bladder. Urine samples were collected into a microcentrifuge tube placed on ice. Once the urine sample was collected, it was heated to 100°C for 90 s to denature enzymes and then cooled on ice and placed in an ultralow (−80°C) freezer until it was analyzed by mass spectrometry.
Purine analysis in urine.
Urine samples were not extracted but were diluted 1:30 with ultrapure water. Heavy-isotope internal standards were added to the diluted samples. Urine samples were analyzed for selected purines using UPLC-MS/MS, as recently described by us (31). Diluted urine samples were injected into a Waters Acquity UPLC system (Milford, MA) equipped with a reversed-phase column (Waters UPLC BEH C18 column, 1.7-µm beads, 2.1 × 150 mm) and analyzed using a TSQ Quantum-Ultra mass spectrometer (Thermo Fisher Scientific, San Jose, CA) operating in the selected reaction monitoring mode with a heated electrospray ionization source. The mobile phase was a linear gradient of 1% acetic acid in water (pH 3; mobile phase A) and 100% methanol (mobile phase B) and was delivered to the column at a flow rate of 300 μl/min. The A-B gradient was as follows: 99.6%-0.4% from 0 to 2 min, 98.0%-2.0% from 2 to 3 min, 85.0%-15.0% from 3 to 4 min, and 99.6%-0.4% from 4 to 6.5 min. Instrument settings were as follows: sample tray temperature, 10°C; column temperature, 50°C; ion spray voltage, 4.0 kV; ion transfer tube temperature, 350°C; source vaporization temperature, 320°C; collision-induced dissociation cell (Q2) gas, argon at 1.5 mTorr; sheath gas, nitrogen at 60 psi; auxiliary gas, nitrogen at 35 psi; resolving quadrupole (Q1/Q3) width, 0.7/0.7 units full-width half-maximum; scan width, 0.6 units; scan time, 0.01 s. The following mass transitions were monitored: 2′,3′-cAMP (330 → 136 m/z), 2′-AMP (348 → 136 m/z), 3′-AMP (348 → 136 m/z), adenosine (268 → 136 m/z), 2′,3′-cGMP (346 → 152 m/z), 3′,5′-cGMP (346 → 152 m/z), 5′-GMP (364 → 152 m/z), 2′-GMP (364 → 152 m/z), 3′-GMP (364 → 152 m/z), guanosine (284 → 152 m/z), 2′,3′-[13C5]cAMP (335 → 136 m/z), 2′-[13C5]AMP (353 → 136 m/z), 3′-[13C5]AMP (353 → 136 m/z), [13C10]adenosine (278 → 141 m/z), 3′,5′-[13C5]cGMP (351 → 152 m/z), 5′-[13C10]GMP (374 → 157 m/z), and [13C10,13N5]guanosine (299 → 162 m/z). The internal standards for quantification of 2′,3′-cAMP, 2′-AMP, 3′-AMP, adenosine, and guanosine were 2′,3′-[13C5]cAMP, 2′-[13C5]AMP, 3′-[13C5]AMP, [13C10]adenosine, and [13C10,13N5]guanosine, respectively. 3′,5′-[13C5]cGMP was used as the internal standard for 2′,3′-cGMP, and 5′-[13C10]GMP was employed as the internal standard for quantifying 2′-GMP and 3′-GMP.
Statistics.
Statistical analysis was conducted using NCSS 12 Statistical Software 2018 (NCSS, Kaysville, UT; ncss.com/software/ncss). Data sets containing only two groups (i.e., those in Figs. 1, 3, and 4) were first tested for normality and equal variances. If the data passed tests for both normality and equal variances, the two groups were compared using an unpaired Student′s t-test. If the data passed normality testing but failed equal variance testing, comparisons were made using the Aspin-Welch t-test for comparing two groups with unequal variances. If the data failed normality testing, comparisons were made using the Mann-Whitney U-test (Wilcoxon’s rank-sum test). Data sets containing two factors with repeated measurements (i.e., those in Figs. 5–7) were first log-transformed to more closely approximate a normal distribution and then analyzed by repeated-measures two-factor ANOVA with post hoc comparisons using a Fisher’s least significant difference test. The criterion of significance was P < 0.05. Values are means ± SE.
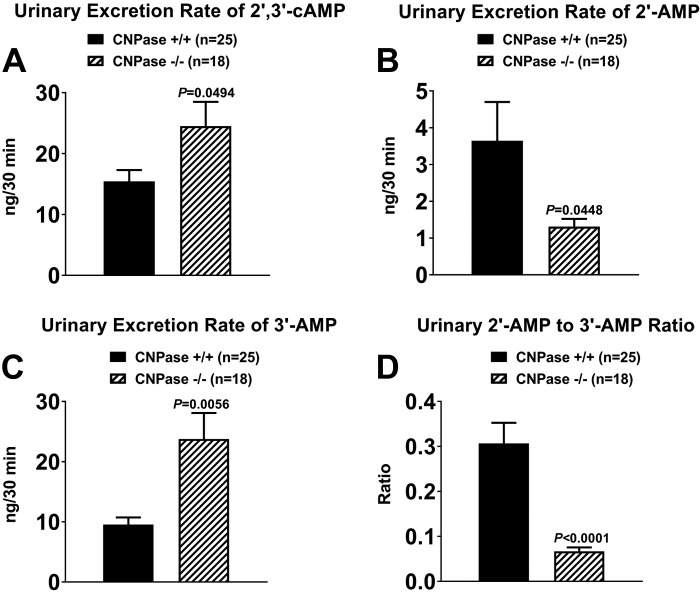
Fig. 1.
A–C: urinary excretion rates of 2′,3′-cAMP, 2′-AMP, and 3′-AMP in 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) wild-type (CNPase+/+) and knockout (CNPase−/−) mice. D: urinary 2′-AMP-to-3′-AMP ratio in CNPase+/+ and CNPase−/− mice. Values are means ± SE.
Fig. 3.
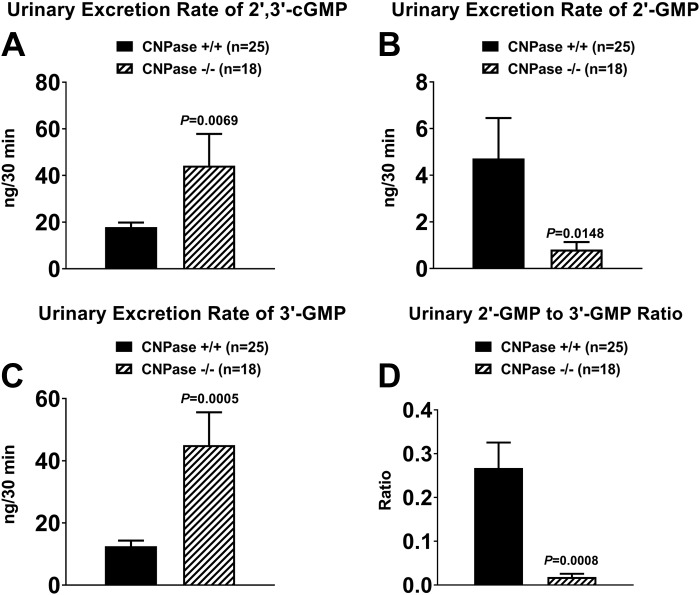
A–C: urinary excretion rates of 2′,3′-cGMP, 2′-GMP, and 3′-GMP in 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) wild-type (CNPase+/+) and knockout (CNPase−/−) mice. D: urinary 2′-GMP-to-3′-GMP ratio in CNPase+/+ and CNPase−/− mice. Values are means ± SE.
Fig. 4.
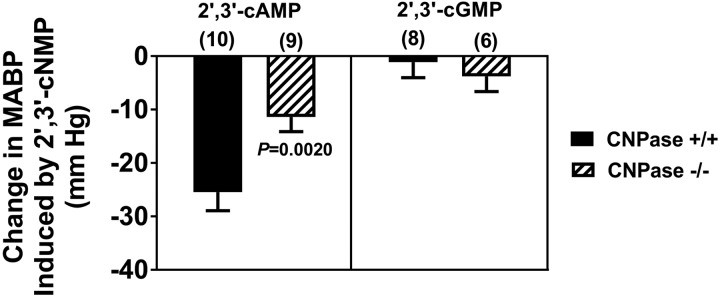
Effects of intravenous infusions of 2′,3′-cAMP and 2′,3′-cGMP, both at 0.3 µmol·kg−1·min−1, on mean arterial blood pressure (MABP) in 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) wild-type (CNPase+/+) and knockout (CNPase−/−) mice. Basal MABPs were as follows: 100 ± 4 mmHg in 2′,3′-cAMP-infused CNPase+/+ mice, 95 ± 3 mmHg in 2′,3′-cAMP-infused CNPase−/− mice, 96 ± 2 mmHg in 2′,3′-cGMP-infused CNPase+/+ mice, and 93 ± 5 mmHg in 2′,3′-cGMP-infused CNPase−/− mice. Values are means ± SE.
Fig. 5.
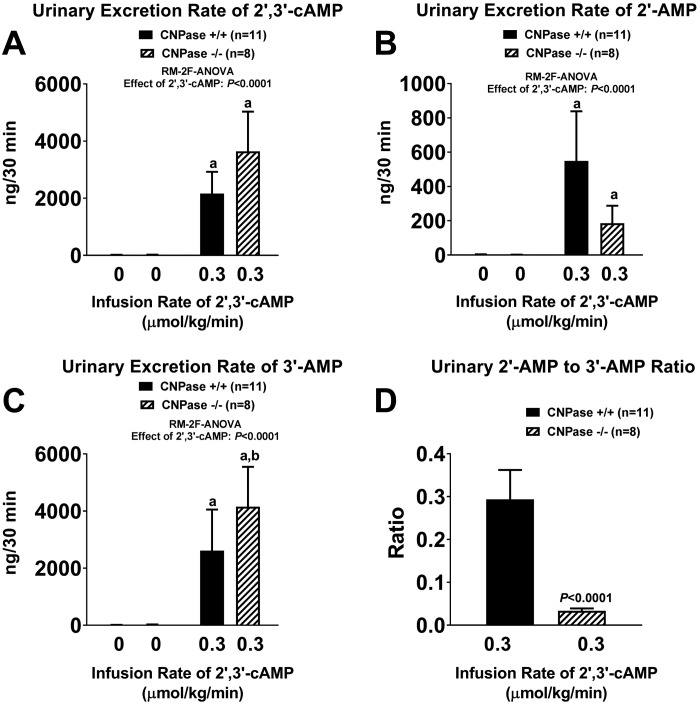
A–C: urinary excretion rates of 2′,3′-cAMP, 2′-AMP, and 3′-AMP before and during intravenous infusion of 2′,3′-cAMP (0.3 µmol·kg−1·min−1) into 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) wild-type (CNPase+/+) and knockout (CNPase−/−) mice. D: urinary 2′-AMP-to-3′-AMP ratio during infusion of 2′,3′-cAMP into CNPase+/+ and CNPase−/− mice. aP < 0.05 vs. basal (0) values, bP < 0.05 vs. corresponding value in CNPase+/+ mice [by repeated-measures 2-factor (RM-2F) ANOVA]. Because the y-axis scale captured the large effects of 2′,3′-cAMP infusions, basal (0) values are near or within the width of the x-axis. Values are means ± SE.
Fig. 7.
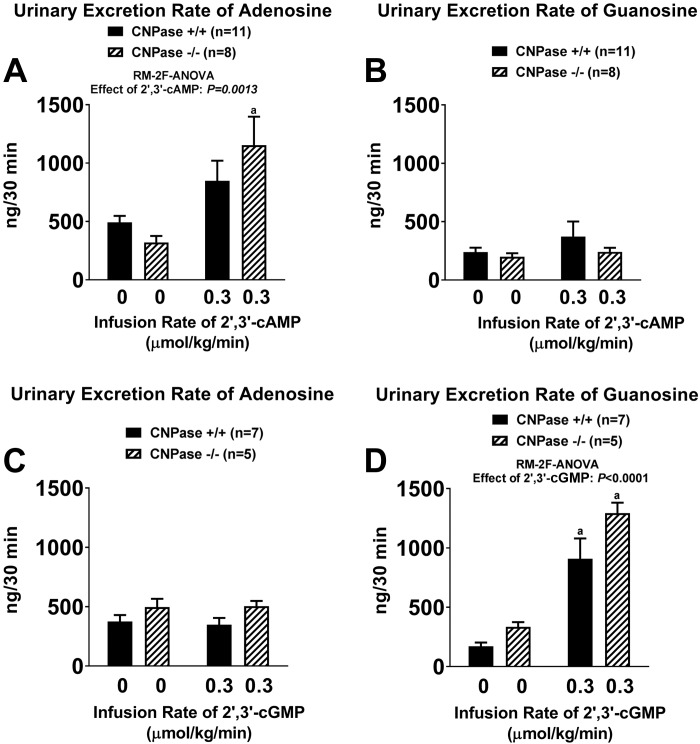
Urinary excretion rates of adenosine (A and C) and guanosine (B and D) before and during intravenous infusion of 2′,3′-cAMP (0.3 µmol·kg−1·min−1; A and B) or 2′,3′-cGMP (0.3 µmol·kg−1·min−1; C and D) into 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) wild-type (CNPase+/+) and knockout (CNPase−/−) mice. aP < 0.05 vs. basal (0) values [by repeated-measures 2-factor (RM-2F) ANOVA]. Values are means ± SE.
RESULTS
Twenty-five CNPase+/+ and 18 CNPase−/− mice were surgically prepared for timed urine collections. Briefly, after a 30-min stabilization period, urine was carefully collected over 30 min into a microcentrifuge tube kept in a cold container. Subsequent to collection, the urine was heat-inactivated to denature any enzymes in the urine that could have metabolized nucleotides and then immediately stored at −80°C until it was assayed by UPLC-MS/MS. Importantly, because the urine was not extracted, postcollection alterations in the composition of the urine were avoided.
As shown in Fig. 1, 2′,3′-cAMP, 2′-AMP, and 3′-AMP were detected in urine samples from CNPase+/+ and CNPase−/− mice. However, urinary excretion of 2′,3′-cAMP was increased by 59% in CNPase−/− compared with CNPase+/+ mice (Fig. 1A). In contrast to 2′,3′-cAMP, urinary excretion of 2′-AMP was reduced by 64% in CNPase−/− compared with CNPase+/+ mice (Fig. 1B). The reduction in urinary excretion of 2′-AMP in CNPase−/− mice was associated with a 149% increase in urinary excretion of 3′-AMP (Fig. 1C). Thus the ratio of urinary excretion of 2′-AMP to 3′-AMP was reduced by 78% in CNPase−/− mice (Fig. 1D).
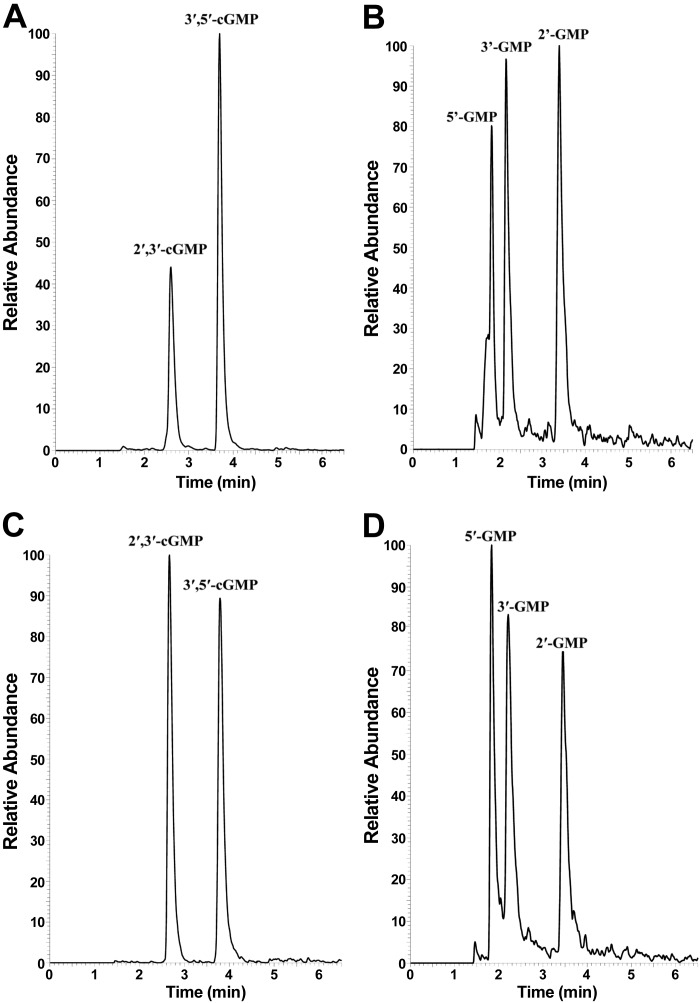
As shown in Fig. 2A, high signal-to-noise-ratio chromatographic peaks were observed when the selected monitoring reaction transition was set to detect specifically 2′,3′-cGMP and 3′,5′-cGMP (i.e., 346 → 152 m/z). Similarly, high signal-to-noise-ratio chromatographic peaks were also observed (Fig. 2D) when the selected monitoring reaction transition was set to detect 5′-GMP, 2′-GMP, and 3′-GMP (i.e., 364 → 152 m/z). Importantly, retention times of the putative signals for 2′,3′-cGMP, 2′-GMP, and 3′-GMP aligned within experimental error to retention times for authentic 2′,3′-cGMP (Fig. 2C), 2′-GMP (Fig. 2D), and 3′-GMP (Fig. 2D), and the patterning for samples and standards was practically identical.
Fig. 2.
Ultraperformance LP-MS/MS chromatograms obtained from urine samples or standards. A: chromatogram obtained from urine samples examined using the selected reaction monitoring (SRM) mass transition tuned for 2′,3′-cGMP and 3′,5′-cGMP (346 → 152 m/z). B: chromatogram obtained from urine samples examined using the SRM mass transition tuned for 5′-GMP, 3′-GMP, and 2′-GMP (364 → 152 m/z). C: chromatogram obtained by UPLC-MS/MS analysis of a standard solution containing authentic 2′,3′-cGMP and 3′,5′-cGMP. D: chromatogram obtained by ultraperformance LC-MS/MS analysis of a standard solution containing authentic 5′-GMP, 3′-GMP, and 2′-GMP. Retention times were as follows: 2.60 and 3.70 min for 2′,3′-cGMP and 3′,5′-cGMP, respectively (A); 1.84, 2.21, and 3.45 min for 5′-GMP, 3′-GMP, and 2′-GMP, respectively (B); 2.67 and 3.80 min for 2′,3′-cGMP and 3′,5′-cGMP, respectively (C); and 1.82, 2.16, and 3.40 min for 5′-GMP, 3′-GMP, and 2′-GMP, respectively (D).
Since CNPase metabolizes all 2′,3′-cNMPs (51), if the chromatographic peaks shown in Fig. 2, A and B, were from 2′,3′-cGMP, 2′-GMP, and 3′-GMP, then urinary excretion of these noncanonical nucleotides in CNPase+/+ vs. CNPase−/− mice should follow the pattern observed for 2′,3′-cAMP, 2′-AMP, and 3′-AMP. Consistent with this concept, urinary excretion of 2′,3′-cGMP was increased by 147% in CNPase−/− compared with CNPase+/+ mice (Fig. 3A), and urinary excretion of 2′-GMP was reduced by 83% in CNPase−/− compared with CNPase+/+ mice (Fig. 3B). Moreover, the reduction in urinary excretion of 2′-GMP in CNPase−/− mice was associated with a 261% increase in urinary excretion of 3′-GMP (Fig. 3C), and the ratio of urinary excretion of 2′-GMP to 3′-GMP was reduced by 93% (Fig. 3D).
Our previous work established the existence of the 2′,3′-cAMP-adenosine pathway, i.e., the metabolism of 2′,3′-cAMP to 2′-AMP and 3′-AMP followed by the conversion of 2′-AMP and 3′-AMP to adenosine (20, 22–24, 27–31, 45, 57, 58). It is conceivable that a similar pathway exits for 2′,3′-cGMP, i.e., 2′,3′-cGMP may be metabolized to 2′-GMP and 3′-GMP, which in turn may be converted to guanosine. To test this notion, in a subset of CNPase+/+ and CNPase−/− mice, we infused 2′,3′-cAMP or 2′,3′-cGMP (both at 0.3 µmol·kg−1·min−1 iv) for 45 min and collected urine samples during the last 30 min of these infusions for subsequent analysis by UPLC-MS/MS. As shown in Fig. 4, 2′,3′-cAMP decreased MABP by ~25 mmHg in CNPase+/+ mice, but only by 11 mmHg in CNPase−/− mice. In contrast, 2′,3′-cGMP had little, if any, effect on MABP.
Intravenous infusions of 2′,3′-cAMP were associated with large increases in urinary excretion of 2′,3′-cAMP (Fig. 5A), 2′-AMP (Fig. 5B), and 3′-AMP (Fig. 5C) in CNPase+/+ and CNPase−/− mice. However, the increases in 2′,3′-cAMP excretion tended to be larger (Fig. 5A) and the increases in urinary 2′-AMP excretion tended to be smaller (Fig. 5B) in CNPase−/− than CNPase+/+ mice, although these changes did not reach statistical significance. Nonetheless, the increase in urinary excretion of 3′-AMP induced by 2′,3′-cAMP was statistically greater in CNPase−/− mice (Fig. 5C), and the urinary 2′-AMP-to-3′-AMP ratio during the infusions of 2′,3′-cAMP were significantly attenuated in CNPase−/− mice (Fig. 5D).
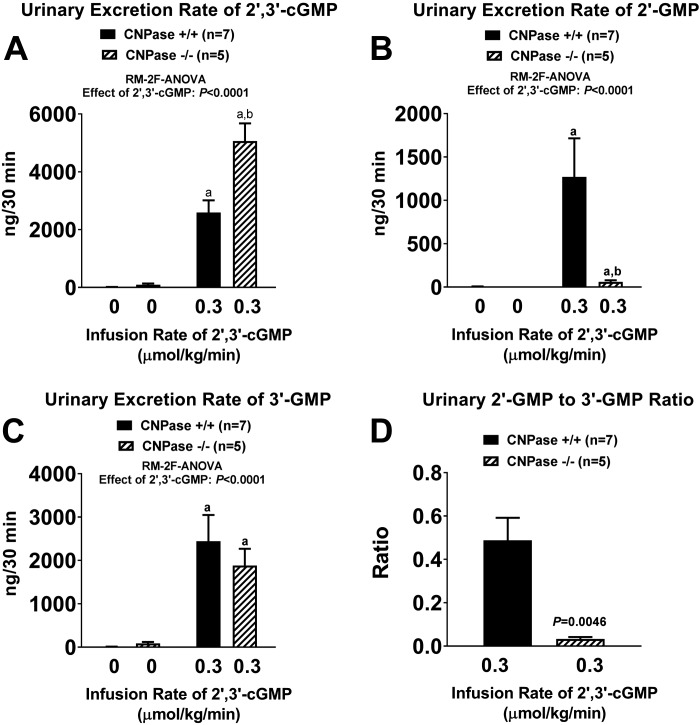
As with 2′,3′-cAMP, intravenous infusions of 2′,3′-cGMP also caused large increases in urinary excretion of 2′,3′-cGMP (Fig. 6A), 2′-GMP (Fig. 6B), and 3′-GMP (Fig. 6C). Infusions of 2′,3′-cGMP increased urinary excretion of 2′,3′-cGMP more in CNPase−/− than CNPase+/+ mice (Fig. 6A), whereas the 2′,3′-cGMP-induced increase in urinary 2′-GMP excretion was nearly abolished in CNPase−/− mice (Fig. 6B). The effect of 2′,3′-cGMP infusions on urinary 3′-GMP excretion was similar in CNPase−/− and CNPase+/+ mice (Fig. 6C). Because 2′,3′-cGMP induced little increase in urinary 2′-GMP in CNPase−/− mice, the urinary 2′-GMP-to-3′-GMP ratio was significantly lower in CNPase−/− than CNPase+/+ mice during 2′,3′-cGMP infusions (Fig. 6D).
Fig. 6.
A–C: urinary excretion rates of 2′,3′-cGMP, 2′-GMP, and 3′-GMP before and during intravenous infusion of 2′,3′-cGMP (0.3 µmol·kg−1·min−1) into 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) wild-type (CNPase+/+) and knockout (CNPase−/−) mice. D: urinary 2′-GMP-to-3′-GMP ratio during infusion of 2′,3′-cGMP into CNPase+/+ and CNPase−/− mice. aP < 0.05 vs. basal (0) values, bP < 0.05 vs. corresponding value in CNPase+/+ mice [by repeated-measures 2-factor (RM-2F) ANOVA]. Because the y-axis scale captured the large effects of 2′,3′-cGMP infusions, basal (0) values are near or within the width of the x-axis. Values are means ± SE.
Consistent with the concept of a 2′,3′-cAMP-adenosine pathway, intravenous infusions of 2′,3′-cAMP significantly increased urinary excretion of adenosine (Fig. 7A), but not guanosine (Fig. 7B). Notably, infusions of 2′,3′-cGMP did not increase urinary excretion of adenosine (Fig. 7C), yet they caused a four- to fivefold increase in urinary excretion of guanosine (Fig. 7D).
The present study was conducted in both female and male mice, and the data were aggregated to increase statistical power. However, it is possible that this approach obscured important effects of sex on these noncanonical nucleotides. To determine whether there were sex-dependent effects, we also examined the data disaggregated according to sex. We did not observe any significant differences between females and males with regard to the urinary excretion rates of any of the purines. As shown in Table 1, the basal urinary excretion rates of 2′,3′-cAMP, 2′-AMP, 3′-AMP, 2′,3′-cGMP, 2′-GMP, and 3′-GMP were similar in female and male CNPase+/+ mice and in female and male CNPase−/− mice.
Table 1.
Urinary excretion rates of 2′,3′-cAMP, 2′-AMP, 3′-AMP, 2′,3′-cGMP, 2′-GMP, and 3′-GMP in female and male mice
| Purine | Female | Male |
|---|---|---|
| CNPase+/+ mice | ||
| 2′,3′-cAMP | 14.4 ± 3.6 | 16.0 ± 2.2 |
| 2′-AMP | 3.0 ± 0.6 | 3.9 ± 1.5 |
| 3′-AMP | 9.2 ± 1.7 | 9.7 ± 1.6 |
| 2′,3′-cGMP | 18.8 ± 2.5 | 17.5 ± 2.6 |
| 2′-GMP | 2.8 ± 1.7 | 5.7 ± 2.5 |
| 3′-GMP | 10.5 ± 2.2 | 13.4 ± 2.5 |
| CNPase−/− mice | ||
| 2′,3′-cAMP | 27.7 ± 7.3 | 22.0 ± 4.3 |
| 2′-AMP | 1.7 ± 0.4 | 1.0 ± 0.2 |
| 3′-AMP | 31.8 ± 8.3 | 17.9 ± 3.9 |
| 2′,3′-cGMP | 40.3 ± 8.0 | 25.4 ± 4.1 |
| 2′-GMP | 0.6 ± 0.6 | 1.0 ± 0.4 |
| 3′-GMP | 39.3 ± 17.4 | 49.3 ± 13.7 |
Values (means ± SE) are expressed in ng/30 min; n = 8 female CNPase+/+ and 8 female CNPase−/− mice and n = 17 male CNPase+/+ and 10 male CNPase−/− mice. CNPase, 2′,3′-cyclic nucleotide 3′-phosphodiesterase. There were no statistically significant differences (by 2-tailed Student’s t-test) between females and males for any of the groups.
DISCUSSION
The first goal of the present study was to determine whether 2′,3′-cGMP exists under physiological conditions. Although there are several reports of 2′,3′-cGMP in extracts of tissues and cells (3, 4, 6, 11, 32, 39, 43, 55, 56), whether 2′,3′-cGMP actually exists in vivo in mammals and under physiological conditions had yet to be established.
The present study provides three lines of evidence that 2′,3′-cGMP exists under physiological conditions. First, when urine was subjected to analysis by UPLC-MS/MS, a strong signal corresponding to the most prominent mass transition for 2′,3′-cGMP (i.e., 346 → 152 m/z) was observed at the chromatographic retention time of authentic 2′,3′-cGMP. Although the mass transition used to detect 2′,3′-cGMP also detected 3′,5′-cGMP, these two cyclic nucleotides were resolved by UPLC with baseline chromatographic separation (~2.6 and 3.7 min for 2′,3′-cGMP and 3′,5′-cGMP, respectively). This rules out the possibility that the signal assigned to 2′,3′-cGMP was instead due to 3′,5′-cGMP. Because the urine was collected by direct cannulation of the bladder and was heat-inactivated and stored at −80°C, it is unlikely that the detected 2′,3′-cGMP was due to bacterial contamination of the urine. Also, because the urine was not extracted, but rather diluted with ultrapure water, it is unlikely that the detected 2′,3′-cGMP was an artifact induced by sample processing. Second, in CNPase−/− mice, the UPLC-MS/MS signal assigned to 2′,3′-cGMP was approximately doubled. CNPase metabolizes the class of 2′,3′-cNMPs to their corresponding 2′-NMPs. Therefore, elimination of CNPase should result in accumulation of 2′,3′-cNMPs. Indeed, in the present study the signals assigned as arising from 2′,3′-cAMP and 2′,3′-cGMP were significantly higher in urine from CNPase−/− than CNPase+/+ mice. This is highly consistent with the conclusion that the signal assigned as arising from 2′,3′-cGMP was indeed due to 2′,3′-cGMP. Third, the signal assigned as arising from 2′,3′-cGMP was massively increased by intravenous infusions of 2′,3′-cGMP. Together, the evidence presented here leaves little doubt that 2′,3′-cGMP is an endogenous cyclic nucleotide that exists in mammals under physiological conditions.
The second goal was to test the hypothesis that there exists in vivo and under physiological conditions a 2′,3′-cGMP-guanosine pathway (2′,3′-cGMP → 2′-GMP/3′-GMP → guanosine) that parallels and coexists with the recently discovered 2′,3′-cAMP-adenosine pathway (2′,3′-cAMP → 2′-AMP/3′-AMP → adenosine). To address this issue, we analyzed urine not only for 2′,3′-cGMP, but also for its downstream metabolites 2′-GMP and 3′-GMP. Here, in urine analyzed by UPLC-MS/MS, we show strong signals corresponding to the most prominent mass transition for 2′-GMP and 3′-GMP (i.e., 364 → 152 m/z) at the chromatographic retention times of authentic 2′-GMP and 3′-GMP. Although the mass transition used to detect 2′-GMP and 3′-GMP also detected 5′-GMP, these three linear nucleotides were resolved by UPLC with baseline chromatographic separation (~1.84, 2.21, and 3.45 min for 5′-GMP, 3′-GMP, and 2′-GMP, respectively). This rules out the possibility that the signals assigned to 3′-GMP and 2′-GMP were instead due to 5′-GMP. Since CNPase metabolizes 2′,3′-cNMPs to their corresponding 2′-NMPs, it would be expected that, in CNPase−/− mice, the metabolism of 2′,3′-cGMP would be diverted from production of 2′-GMP (which depends on CNPase activity) toward generation of 3′-GMP (which does not depend on CNPase activity). Therefore, elimination of CNPase should result in accumulation of 3′-GMP, depletion of 2′-GMP, and suppression of the urinary ratio of 2′-GMP to 3′-GMP. Indeed, in the present study in CNPase−/− mice, the UPLC-MS/MS signal assigned to 2′-GMP was reduced by 83%, the signal assigned to 3′-GMP was increased by 261%, and the ratio of urinary 2′-GMP to 3′-GMP was reduced by 93%. These findings are highly consistent with the conclusion that the signals assigned as arising from 2′-GMP and 3′-GMP were indeed due to these compounds. We also observed the nucleoside guanosine in urine from both CNPase+/+ and CNPase−/− mice. Together, these data show that urine contains 2′,3′-cGMP, 2′-GMP, 3′-GMP, and guanosine, i.e., all the components of the putative 2′,3′-cGMP-guanosine pathway.
To further test the physiological existence of the 2′,3′-cGMP-guanosine pathway, we infused into mice a low dose of exogenous 2′,3′-cGMP and collected urine for measurement of 2′,3′-cGMP, 2′-GMP, 3′-GMP, and guanosine. As expected, infusions of 2′,3′-cGMP increased urinary excretion of 2′-GMP, 3′-GMP, and guanosine. Notably, the 2′,3′-cGMP-induced increases in urinary 2′,3′-cGMP and 2′-GMP were significantly increased and attenuated, respectively, in CNPase−/− compared with CNPase+/+ mice. This indicates an active role for CNPase in the 2′,3′-cGMP-guanosine pathway. However, infusions of 2′,3′-cGMP increased urinary guanosine excretion similarly in CNPase+/+ and CNPase−/− mice. This suggests that, in the urinary compartment, when 2′,3′-cGMP levels are high, production of guanosine from 2′,3′-cGMP can be maintained via 3′-GMP, even when 2′-GMP production is compromised by knockout of CNPase.
Notably, intravenous infusions of 2′,3′-cAMP, but not 2′,3′-cGMP, deceased MABP in mice. Moreover, the hemodynamic response to 2′,3′-cAMP was attenuated in CNPase−/− mice. We previously reported that, in rats, 2′,3′-cAMP induces marked hypotension via its conversion to adenosine (26). Therefore, it is likely that the hypotensive response to 2′,3′-cAMP in mice observed here was due to conversion of 2′,3′-cAMP to adenosine. This suggests that, with regard to the 2′,3′-cAMP-adenosine pathway, CNPase plays an important role in the production of adenosine within the cardiovascular system. However, knockout of CNPase did not affect the increase in urinary excretion of adenosine induced by infusions of 2′,3′-cAMP. This suggests that in the urinary compartment, when 2′,3′-cAMP levels are high, production of adenosine from 2′,3′-cAMP can be maintained via 3′-AMP, even when 2′-AMP production is compromised by knockout of CNPase. The lack of effect of 2′,3′-cGMP on MABP is consistent with the fact that guanosine, unlike adenosine, does not cause hypotension.
What is the significance of 2′,3′-cGMP? Emerging evidence shows that 2′,3′-cAMP, but not 2′,3′-cGMP, promotes opening of mitochondrial permeability transition pores (1, 2) in mammalian mitochondria. Also, in plant cells, 2′,3′-cAMP promotes stress granule formation, in part, by binding to RNA-binding protein 47b, whereas 2′,3′-cGMP only weakly binds to RNA-binding protein 47b (39). However, given the fact that functional roles for 2′,3′-cAMP have been discovered, it is likely that biological roles for 2′,3′-cGMP also will eventually be discovered.
One possible biological role for 2′,3′-cGMP is in the production of guanosine via the 2′,3′-cGMP-guanosine pathway. Guanosine is now considered a key tissue-protective compound: guanosine per se is both neuroprotective (50) and renoprotective (33). As reviewed by Schmidt et al. (50), guanosine provides neuroprotection via multiple mechanisms, including 1) enhancement of glutamate uptake (12, 40, 41), 2) activation of MAPK pathways (54), 3) engagement of the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase-3β pathway (44), and 4) inhibition of inducible nitric oxide synthase (44). Also guanosine, like inosine, has strong anti-inflammatory activity and reduces oxidative stress (5, 8, 9, 13, 14, 42, 46–48, 52, 53). In addition, our studies show that guanosine increases extracellular levels of adenosine by blocking the disposition of extracellular adenosine (18, 19, 21, 25). Since adenosine is cardioprotective (7), renoprotective (59), and neuroprotective (17, 34–38), guanosine may be tissue-protective, in part, by activating adenosine receptors. Indeed, the neuroprotective effects of systemically administered guanosine in a rat model of brain injury are mediated by adenosinergic receptors (10). It is unknown whether guanosine receptors exist and precisely how guanosine alters extracellular adenosine levels.
Perspectives and Significance
Here we provide strong evidence for the physiological existence of 2′,3′-cGMP and its downstream metabolites 2′-GMP and 3′-GMP. Moreover, we show that 2′,3′-cGMP is metabolized to guanosine, thus identifying a new biochemical pathway (i.e., the 2′,3′-cGMP-guanosine pathway). We propose that the 2′,3′-cGMP-guanosine pathway (reported here for the first time) and the 2′,3′-cAMP-adenosine pathway (previously reported and confirmed here) may act in concert to provide organ protection. Since formation of 2′,3′-cNMPs is triggered by tissue injury, leading to RNA degradation (30), it is conceivable that the simultaneous activation of the 2′,3′-cGMP-guanosine and 2′,3′-cAMP-adenosine pathways provides tissue protection via guanosine, adenosine, and the interaction between guanosine and adenosine. This concept is summarized in Fig. 8. Future research in this area should focus on the roles of both the 2′,3′-cAMP-adenosine pathway and the 2′,3′-cGMP-guanosine pathway in physiology.
Fig. 8.
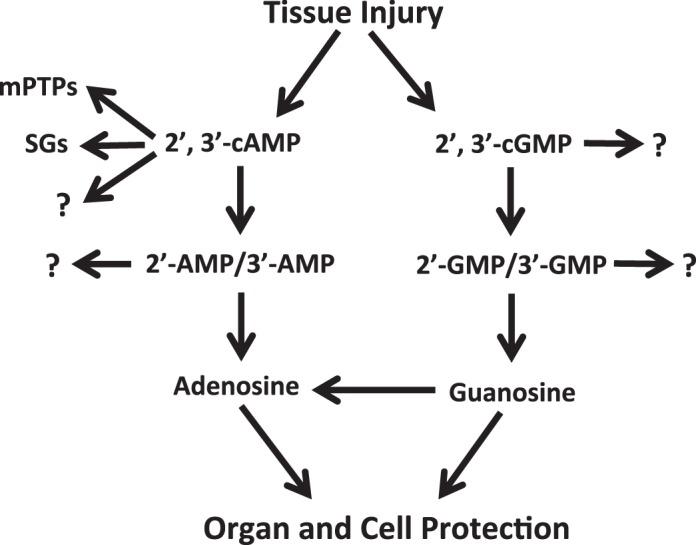
Summary of the possible biological roles of the 2′,3′-cGMP-guanosine and 2′,3′-cAMP-adenosine pathways. Tissue injury is known to trigger degradation of RNA, which is the main mechanism for production of nucleoside 2′,3′-cyclic monophosphates. 2′,3′-cAMP is known to facilitate opening of mitochondrial permeability transition pores (mPTPs) and, thereby, induce mitophagy and apoptosis (which can be beneficial or harmful, depending on context). In addition, 2′,3′-cAMP is known to bind to proteins that form stress granules (SGs) and, thereby, triggers SG formation (a protective mechanism). 2′,3′-cAMP may have yet additional unknown (?) effects. Although 2′,3′-cGMP does not significantly affect mPTPs and SGs, it is likely that this noncanonical nucleotide has other unknown (?) direct effects on cell biology. Both 2′,3′-cAMP and 2′,3′-cGMP are converted to their corresponding monophosphate metabolites, which may also have unknown (?) biological effects. 2′-AMP and 3′-AMP are converted to adenosine, whereas 2′-GMP and 3′-GMP are metabolized to guanosine. Both adenosine and guanosine are known to provide organ and cell protection. Additionally, guanosine is known to elevate extracellular levels of adenosine.
GRANTS
This work was supported by National Institutes of Health Grants NS-087978, DK-091190, HL-109002, HL-069846, DK-068575, and DK-079307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.K.J., T.C.J., and P.M.K. conceived and designed research; E.K.J., Z.M., and K.J.-F. analyzed data; E.K.J., T.C.J., and P.M.K. interpreted results of experiments; E.K.J. prepared figures; E.K.J. drafted manuscript; E.K.J., T.C.J., and P.M.K. edited and revised manuscript; E.K.J., Z.M., K.J.-F., T.C.J., and P.M.K. approved final version of manuscript; Z.M. and K.J.-F. performed experiments.
REFERENCES
- 1.Azarashvili T, Krestinina O, Galvita A, Grachev D, Baburina Y, Stricker R, Evtodienko Y, Reiser G. Ca2+-dependent permeability transition regulation in rat brain mitochondria by 2′,3′-cyclic nucleotides and 2′,3′-cyclic nucleotide 3′-phosphodiesterase. Am J Physiol Cell Physiol 296: C1428–C1439, 2009. doi: 10.1152/ajpcell.00006.2009. [DOI] [PubMed] [Google Scholar]
- 2.Azarashvili T, Stricker R, Reiser G. The mitochondria permeability transition pore complex in the brain with interacting proteins—promising targets for protection in neurodegenerative diseases. Biol Chem 391: 619–629, 2010. doi: 10.1515/bc.2010.070. [DOI] [PubMed] [Google Scholar]
- 3.Bähre H, Kaever V. Measurement of 2′,3′-cyclic nucleotides by liquid chromatography-tandem mass spectrometry in cells. J Chromatogr B Analyt Technol Biomed Life Sci 964: 208–211, 2014. doi: 10.1016/j.jchromb.2014.02.046. [DOI] [PubMed] [Google Scholar]
- 4.Belghit I, Rasinger JD, Heesch S, Biancarosa I, Liland N, Torstensen B, Waagbø R, Lock E-J, Bruckner CG. In-depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res 26: 240–249, 2017. doi: 10.1016/j.algal.2017.08.001. [DOI] [Google Scholar]
- 5.Bellaver B, Souza DG, Bobermin LD, Gonçalves CA, Souza DO, Quincozes-Santos A. Guanosine inhibits LPS-induced pro-inflammatory response and oxidative stress in hippocampal astrocytes through the heme oxygenase-1 pathway. Purinergic Signal 11: 571–580, 2015. doi: 10.1007/s11302-015-9475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burhenne H, Tschirner S, Seifert R, Kaever V. Identification and quantitation of 2′,3′-cGMP in murine tissues. BMC Pharmacol Toxicol 14: 12, 2013. doi: 10.1186/2050-6511-14-S1-P12.23402420 [DOI] [Google Scholar]
- 7.Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol 103: 203–215, 2008. doi: 10.1007/s00395-007-0687-7. [DOI] [PubMed] [Google Scholar]
- 8.Dal-Cim T, Ludka FK, Martins WC, Reginato C, Parada E, Egea J, López MG, Tasca CI. Guanosine controls inflammatory pathways to afford neuroprotection of hippocampal slices under oxygen and glucose deprivation conditions. J Neurochem 126: 437–450, 2013. doi: 10.1111/jnc.12324. [DOI] [PubMed] [Google Scholar]
- 9.Dalla Corte CL, Bastos LL, Dobrachinski F, Rocha JB, Soares FA. The combination of organoselenium compounds and guanosine prevents glutamate-induced oxidative stress in different regions of rat brains. Brain Res 1430: 101–111, 2012. doi: 10.1016/j.brainres.2011.10.049. [DOI] [PubMed] [Google Scholar]
- 10.Dobrachinski F, Gerbatin RR, Sartori G, Golombieski RM, Antoniazzi A, Nogueira CW, Royes LF, Fighera MR, Porciuncula LO, Cunha RA, Soares FAA. Guanosine attenuates behavioral deficits after traumatic brain injury by modulation of adenosinergic receptors. Mol Neurobiol 56: 3145–3158, 2019. doi: 10.1007/s12035-018-1296-1. [DOI] [PubMed] [Google Scholar]
- 11.Fontaine BM, Martin KS, Garcia-Rodriguez JM, Jung C, Briggs L, Southwell JE, Jia X, Weinert EE. RNase I regulates Escherichia coli 2′,3′-cyclic nucleotide monophosphate levels and biofilm formation. Biochem J 475: 1491–1506, 2018. doi: 10.1042/BCJ20170906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frizzo ME, Lara DR, Prokopiuk AS, Vargas CR, Salbego CG, Wajner M, Souza DO. Guanosine enhances glutamate uptake in brain cortical slices at normal and excitotoxic conditions. Cell Mol Neurobiol 22: 353–363, 2002. doi: 10.1023/A:1020728203682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansel G, Ramos DB, Delgado CA, Souza DG, Almeida RF, Portela LV, Quincozes-Santos A, Souza DO. The potential therapeutic effect of guanosine after cortical focal ischemia in rats [Electronic Resource]. PLoS One 9: e90693, 2014. doi: 10.1371/journal.pone.0090693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansel G, Tonon AC, Guella FL, Pettenuzzo LF, Duarte T, Duarte MMMF, Oses JP, Achaval M, Souza DO. Guanosine protects against cortical focal ischemia. Involvement of inflammatory response. Mol Neurobiol 52: 1791–1803, 2015. doi: 10.1007/s12035-014-8978-0. [DOI] [PubMed] [Google Scholar]
- 15.Jackson EK. The 2′,3′-cAMP-adenosine pathway. Am J Physiol Renal Physiol 301: F1160–F1167, 2011. doi: 10.1152/ajprenal.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson EK. Discovery and roles of 2′,3′-cAMP in biological systems. In: Handbook of Experimental Pharmacology, edited by Seifert R. Cham, Switzerland: Springer, 2017, p. 229–252. [DOI] [PubMed] [Google Scholar]
- 17.Jackson EK, Boison D, Schwarzschild MA, Kochanek PM. Purines: forgotten mediators in traumatic brain injury. J Neurochem 137: 142–153, 2016. doi: 10.1111/jnc.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson EK, Cheng D, Jackson TC, Verrier JD, Gillespie DG. Extracellular guanosine regulates extracellular adenosine levels. Am J Physiol Cell Physiol 304: C406–C421, 2013. doi: 10.1152/ajpcell.00212.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson EK, Cheng D, Mi Z, Gillespie DG. Guanosine regulates adenosine levels in the kidney. Physiol Rep 2: e12028, 2014. doi: 10.14814/phy2.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson EK, Gillespie DG. Extracellular 2′,3′-cAMP-adenosine pathway in proximal tubular, thick ascending limb, and collecting duct epithelial cells. Am J Physiol Renal Physiol 304: F49–F55, 2013. doi: 10.1152/ajprenal.00571.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson EK, Gillespie DG. Regulation of cell proliferation by the guanosine-adenosine mechanism: role of adenosine receptors. Physiol Rep 1: e00024, 2013. doi: 10.1002/phy2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson EK, Gillespie DG, Dubey RK. 2′-AMP and 3′-AMP inhibit proliferation of preglomerular vascular smooth muscle cells and glomerular mesangial cells via A2B receptors. J Pharmacol Exp Ther 337: 444–450, 2011. doi: 10.1124/jpet.110.178137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson EK, Gillespie DG, Mi Z, Cheng D, Bansal R, Janesko-Feldman K, Kochanek PM. Role of 2′,3′-cyclic nucleotide 3′-phosphodiesterase in the renal 2′,3′-cAMP-adenosine pathway. Am J Physiol Renal Physiol 307: F14–F24, 2014. doi: 10.1152/ajprenal.00134.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson EK, Menshikova EV, Mi Z, Verrier JD, Bansal R, Janesko-Feldman K, Jackson TC, Kochanek PM. Renal 2′,3′-cyclic nucleotide 3′-phosphodiesterase is an important determinant of AKI severity after ischemia-reperfusion. J Am Soc Nephrol 27: 2069–2081, 2016. doi: 10.1681/ASN.2015040397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson EK, Mi Z. The guanosine-adenosine interaction exists in vivo. J Pharmacol Exp Ther 350: 719–726, 2014. doi: 10.1124/jpet.114.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson EK, Mi Z. In vivo cardiovascular pharmacology of 2′,3′-cAMP, 2′-AMP, and 3′-AMP in the rat. J Pharmacol Exp Ther 346: 190–200, 2013. doi: 10.1124/jpet.113.205757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson EK, Ren J, Cheng D, Mi Z. Extracellular cAMP-adenosine pathways in the mouse kidney. Am J Physiol Renal Physiol 301: F565–F573, 2011. doi: 10.1152/ajprenal.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson EK, Ren J, Gillespie DG. 2′,3′-cAMP, 3′-AMP, and 2′-AMP inhibit human aortic and coronary vascular smooth muscle cell proliferation via A2B receptors. Am J Physiol Heart Circ Physiol 301: H391–H401, 2011. doi: 10.1152/ajpheart.00336.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson EK, Ren J, Gillespie DG, Dubey RK. Extracellular 2,3-cyclic adenosine monophosphate is a potent inhibitor of preglomerular vascular smooth muscle cell and mesangial cell growth [corrected]. Hypertension 56: 151–158, 2010. [Erratum in Hypertension 56: e25, 2010.] doi: 10.1161/HYPERTENSIONAHA.110.152454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem 284: 33097–33106, 2009. doi: 10.1074/jbc.M109.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson TC, Kotermanski SE, Kochanek PM, Jackson EK. Oxidative stress induces release of 2′-AMP from microglia. Brain Res 1706: 101–109, 2019. doi: 10.1016/j.brainres.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia X, Fontaine BM, Strobel F, Weinert EE. A facile and sensitive method for quantification of cyclic nucleotide monophosphates in mammalian organs: basal levels of eight cNMPs and identification of 2′,3′-cIMP. Biomolecules 4: 1070–1092, 2014. doi: 10.3390/biom4041070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly KJ, Plotkin Z, Dagher PC. Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury. J Clin Invest 108: 1291–1298, 2001. doi: 10.1172/JCI13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kochanek PM, Clark RS, Obrist WD, Carcillo JA, Jackson EK, Mi Z, Wisniewski SR, Bell MJ, Marion DW. The role of adenosine during the period of delayed cerebral swelling after severe traumatic brain injury in humans. Acta Neurochir Suppl (Wien) 70, Suppl: 109–111, 1997. doi: 10.1007/978-3-7091-6837-0_34. [DOI] [PubMed] [Google Scholar]
- 35.Kochanek PM, Dixon CE, Shellington DK, Shin SS, Bayır H, Jackson EK, Kagan VE, Yan HQ, Swauger PV, Parks SA, Ritzel DV, Bauman R, Clark RS, Garman RH, Bandak F, Ling G, Jenkins LW. Screening of biochemical and molecular mechanisms of secondary injury and repair in the brain after experimental blast-induced traumatic brain injury in rats. J Neurotrauma 30: 920–937, 2013. doi: 10.1089/neu.2013.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kochanek PM, Hendrich KS, Jackson EK, Wisniewski SR, Melick JA, Shore PM, Janesko KL, Zacharia L, Ho C. Characterization of the effects of adenosine receptor agonists on cerebral blood flow in uninjured and traumatically injured rat brain using continuous arterial spin-labeled magnetic resonance imaging. J Cereb Blood Flow Metab 25: 1596–1612, 2005. doi: 10.1038/sj.jcbfm.9600154. [DOI] [PubMed] [Google Scholar]
- 37.Kochanek PM, Hendrich KS, Robertson CL, Williams DS, Melick JA, Ho C, Marion DW, Jackson EK. Assessment of the effect of 2-chloroadenosine in normal rat brain using spin-labeled MRI measurement of perfusion. Magn Reson Med 45: 924–929, 2001. doi: 10.1002/mrm.1123. [DOI] [PubMed] [Google Scholar]
- 38.Kochanek PM, Vagni VA, Janesko KL, Washington CB, Crumrine PK, Garman RH, Jenkins LW, Clark RSB, Homanics GE, Dixon CE, Schnermann J, Jackson EK. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab 26: 565–575, 2006. doi: 10.1038/sj.jcbfm.9600218. [DOI] [PubMed] [Google Scholar]
- 39.Kosmacz M, Luzarowski M, Kerber O, Leniak E, Gutiérrez-Beltrán E, Moreno JC, Gorka M, Szlachetko J, Veyel D, Graf A, Skirycz A. Interaction of 2′,3′-cAMP with Rbp47b plays a role in stress granule formation. Plant Physiol 177: 411–421, 2018. doi: 10.1104/pp.18.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanznaster D, Mack JM, Coelho V, Ganzella M, Almeida RF, Dal-Cim T, Hansel G, Zimmer ER, Souza DO, Prediger RD, Tasca CI. Guanosine prevents anhedonic-like behavior and impairment in hippocampal glutamate transport following amyloid-β1–40 administration in mice. Mol Neurobiol 54: 5482–5496, 2017. doi: 10.1007/s12035-016-0082-1. [DOI] [PubMed] [Google Scholar]
- 41.Lara DR, Schmidt AP, Frizzo ME, Burgos JS, Ramírez G, Souza DO. Effect of orally administered guanosine on seizures and death induced by glutamatergic agents. Brain Res 912: 176–180, 2001. doi: 10.1016/S0006-8993(01)02734-2. [DOI] [PubMed] [Google Scholar]
- 42.Li DW, Yao M, Dong YH, Tang MN, Chen W, Li GR, Sun BQ. Guanosine exerts neuroprotective effects by reversing mitochondrial dysfunction in a cellular model of Parkinson’s disease. Int J Mol Med 34: 1358–1364, 2014. doi: 10.3892/ijmm.2014.1904. [DOI] [PubMed] [Google Scholar]
- 43.Liu A, Yu Y, Sheng Q, Zheng XY, Yang JY, Li PY, Shi M, Zhou BC, Zhang YZ, Chen XL. Identification of four kinds of 2′,3′-cNMPs in Escherichia coli and a method for their preparation. ACS Chem Biol 11: 2414–2419, 2016. doi: 10.1021/acschembio.6b00426. [DOI] [PubMed] [Google Scholar]
- 44.Molz S, Dal-Cim T, Budni J, Martín-de-Saavedra MD, Egea J, Romero A, del Barrio L, Rodrigues AL, López MG, Tasca CI. Neuroprotective effect of guanosine against glutamate-induced cell death in rat hippocampal slices is mediated by the phosphatidylinositol-3 kinase/Akt/glycogen synthase kinase 3β pathway activation and inducible nitric oxide synthase inhibition. J Neurosci Res 89: 1400–1408, 2011. doi: 10.1002/jnr.22681. [DOI] [PubMed] [Google Scholar]
- 45.Newell EA, Exo JL, Verrier JD, Jackson TC, Gillespie DG, Janesko-Feldman K, Kochanek PM, Jackson EK. 2′,3′-cAMP, 3′-AMP, 2′-AMP and adenosine inhibit TNF-α and CXCL10 production from activated primary murine microglia via A2A receptors. Brain Res 1594: 27–35, 2015. doi: 10.1016/j.brainres.2014.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paniz LG, Calcagnotto ME, Pandolfo P, Machado DG, Santos GF, Hansel G, Almeida RF, Bruch RS, Brum LM, Torres FV, de Assis AM, Rico EP, Souza DO. Neuroprotective effects of guanosine administration on behavioral, brain activity, neurochemical and redox parameters in a rat model of chronic hepatic encephalopathy. Metab Brain Dis 29: 645–654, 2014. doi: 10.1007/s11011-014-9548-x. [DOI] [PubMed] [Google Scholar]
- 47.Quincozes-Santos A, Bobermin LD, de Souza DG, Bellaver B, Gonçalves CA, Souza DO. Gliopreventive effects of guanosine against glucose deprivation in vitro. Purinergic Signal 9: 643–654, 2013. doi: 10.1007/s11302-013-9377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quincozes-Santos A, Bobermin LD, Souza DG, Bellaver B, Gonçalves CA, Souza DO. Guanosine protects C6 astroglial cells against azide-induced oxidative damage: a putative role of heme oxygenase 1. J Neurochem 130: 61–74, 2014. doi: 10.1111/jnc.12694. [DOI] [PubMed] [Google Scholar]
- 49.Ren J, Mi Z, Stewart NA, Jackson EK. Identification and quantification of 2′,3′-cAMP release by the kidney. J Pharmacol Exp Ther 328: 855–865, 2009. doi: 10.1124/jpet.108.146712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt AP, Lara DR, Souza DO. Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol Ther 116: 401–416, 2007. doi: 10.1016/j.pharmthera.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Sprinkle TJ. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit Rev Neurobiol 4: 235–301, 1989. [PubMed] [Google Scholar]
- 52.Tarozzi A, Merlicco A, Morroni F, Bolondi C, Di Iorio P, Ciccarelli R, Romano S, Giuliani P, Hrelia P. Guanosine protects human neuroblastoma cells from oxidative stress and toxicity induced by amyloid-β peptide oligomers. J Biol Regul Homeost Agents 24: 297–306, 2010. [PubMed] [Google Scholar]
- 53.Thomaz DT, Dal-Cim TA, Martins WC, Cunha MP, Lanznaster D, de Bem AF, Tasca CI. Guanosine prevents nitroxidative stress and recovers mitochondrial membrane potential disruption in hippocampal slices subjected to oxygen/glucose deprivation. Purinergic Signal 12: 707–718, 2016. doi: 10.1007/s11302-016-9534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomaselli B, Podhraski V, Heftberger V, Böck G, Baier-Bitterlich G. Purine nucleoside-mediated protection of chemical hypoxia-induced neuronal injuries involves p42/44 MAPK activation. Neurochem Int 46: 513–521, 2005. doi: 10.1016/j.neuint.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Van Damme T, Blancquaert D, Couturon P, Van Der Straeten D, Sandra P, Lynen F. Wounding stress causes rapid increase in concentration of the naturally occurring 2′,3′-isomers of cyclic guanosine- and cyclic adenosine monophosphate (cGMP and cAMP) in plant tissues. Phytochemistry 103: 59–66, 2014. doi: 10.1016/j.phytochem.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 56.Van Damme T, Zhang Y, Lynen F, Sandra P. Determination of cyclic guanosine- and cyclic adenosine monophosphate (cGMP and cAMP) in human plasma and animal tissues by solid phase extraction on silica and liquid chromatography-triple quadrupole mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 909: 14–21, 2012. doi: 10.1016/j.jchromb.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Verrier JD, Exo JL, Jackson TC, Ren J, Gillespie DG, Dubey RK, Kochanek PM, Jackson EK. Expression of the 2′,3′-cAMP-adenosine pathway in astrocytes and microglia. J Neurochem 118: 979–987, 2011. doi: 10.1111/j.1471-4159.2011.07392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verrier JD, Jackson TC, Bansal R, Kochanek PM, Puccio AM, Okonkwo DO, Jackson EK. The brain in vivo expresses the 2′,3′-cAMP-adenosine pathway. J Neurochem 122: 115–125, 2012. doi: 10.1111/j.1471-4159.2012.07705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yap SC, Lee HT. Adenosine and protection from acute kidney injury. Curr Opin Nephrol Hypertens 21: 24–32, 2012. doi: 10.1097/MNH.0b013e32834d2ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]