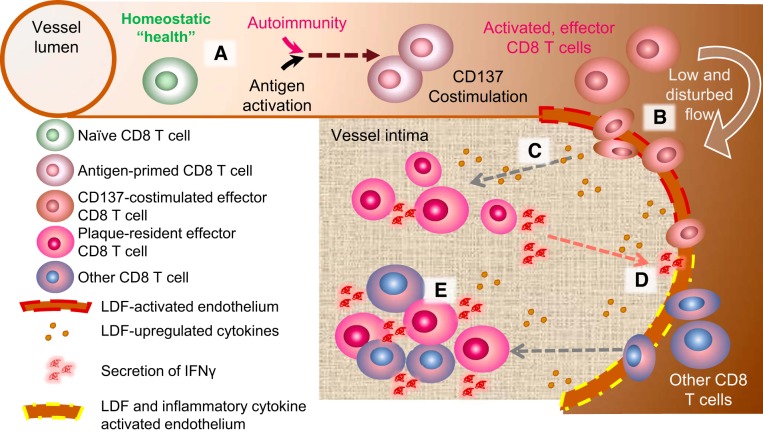
Fig. 9.
Proposed model for how CD137 costimulation promotes the seeding of activated effector CD8 T cells into nascent atherogenic foci. A: under conditions of homeostatic health, naive CD8 T cells do not readily infiltrate the vessel wall, even at sites of low and disturbed flow (LDF)-activated endothelium, where adhesion receptors or chemokines are upregulated (red dashed line). B: increased circulation of effector CD8 T cells by CD137 costimulation prompts their infiltration at LDF foci independent of cognate TCR recognition. C: LDF activates intimal cells at atherogenic foci to secrete cytokines, such as IL-36γ, that promote innate-like secretion of IFNγ from infiltrated CD8 T cells. D: proinflammatory cytokines produced in C further alter the plaque microenvironment, generating an intimal niche (yellow dashed line) that is permissive to infiltration of other CD8 T cells in circulation. E: together, the initial seeding of CD137-stimulated effector CD8 T cells and the subsequent infiltration of other CD8 T cells constitute a plaque with increased inflammatory potential, potentially resulting in accelerated atherosclerotic pathology and vascular events.