Fig. 6.
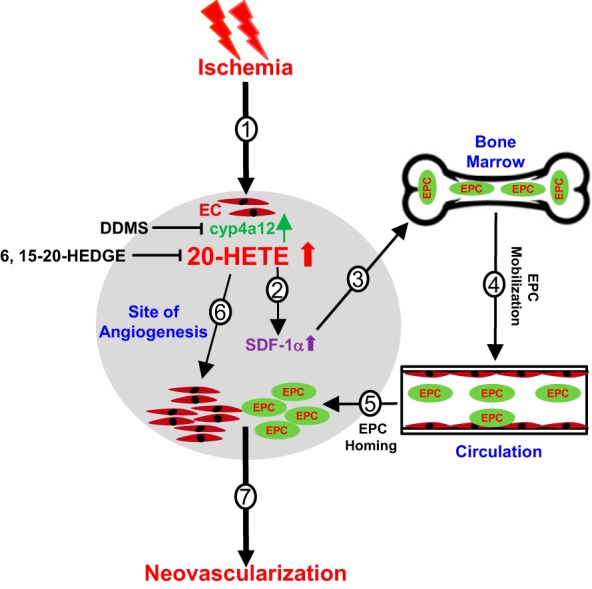
Schematics by which CYP4A/20-hydroxyeicosatetraenoic acid (20-HETE) regulates ischemia-induced neovascularization processes via endothelial progenitor cells (EPCs) and preexisting endothelial cells (ECs). The CYP4A/20-HETE axis regulates ischemic neovascularization via a multistep process. Ischemia induces an increase in cyp4a12 expression and 20-HETE production by injured endothelium (step 1); increases in 20-HETE stimulates the production and release of stromal cell-derived factor-1α (SDF-1α) (step 2); SDF-1α in turn signals EPC mobilization from bone marrow to circulation (steps 3 and 4); EPCs then home toward the target sites of angiogenesis (step 5). In parallel, 20-HETE increases secondary to ischemia at the local endothelium and leads to upregulated preexisting EC response, such as EC proliferation in autocrine and paracrine manners. The hypoxic and ischemic conditions at the target sites may further reinforce the level of 20-HETE by amplifying additional angiogenic signals through preexisting EC networks (step 6). Thus, the combined vasculogenic effects via EPC and angiogenic effects via a preexisting EC network by 20-HETE collectively resulted in increased neovascularization in response to ischemia.
