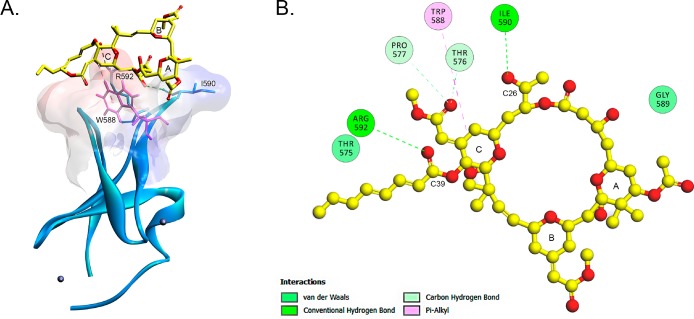
Figure 9.
Interaction of bryostatin 1 and Munc13-1. (A) Rim of the active site of the Munc13-1 C1 domain and the docking pose of bryostatin 1 on the Munc13-1 C1 domain. The ribbon structure of the C1 domain of Munc 13-1 with the two Zn ions (balls) is shown. The surface of the structure is colored by hydrophobicity according to the Kyte–Doolittle scale. Blue indicates strong hydrophobicity, and red indicates weak hydrophobicity. The line structure represents bryostatin 1 (yellow). (B) Bryostatin 1 forms two hydrogen bonds (green) and one hydrophobic interaction (magenta) with the residues of the two C1 domain loops.