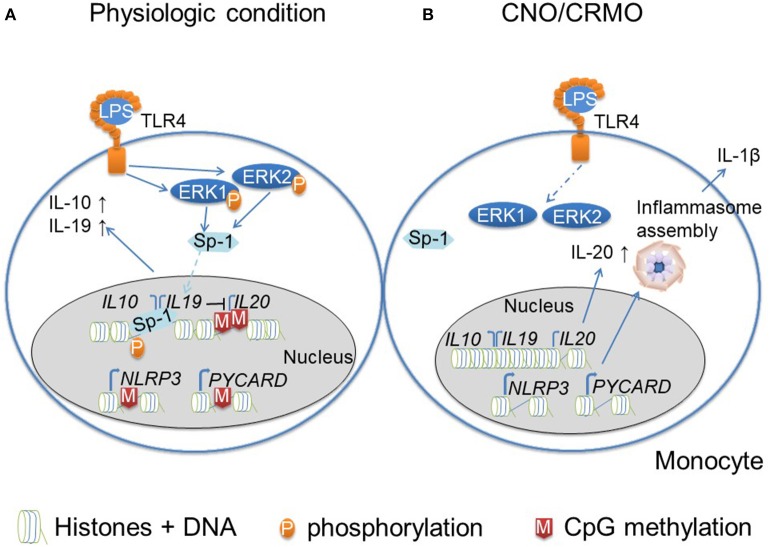
Figure 2.
Monocytes from CNO/CRMO patients are epigenetically primed for inflammation. (A) In response to TLR4 activation (with lipopolysaccharide; LPS) monocytes from healthy individuals phosphorylate mitogen activated protein kinases (MAPK) extracellular signal reactive kinases (ERK)1 and 2. Kinases activate the transcriptional regulatory factor signaling protein Sp-1, which results in its translocation into the nucleus. Furthermore, ERK1/2 contribute to histone H3 phosphorylation at serine residue 10 (H3S10) resulting in an open chromatin structure at IL10 and IL19. These events together result in trans-activation of IL10 and IL19. Immune regulatory cytokine expression (IL-10 and IL-19) negatively affect the expression of the pro-inflammatory IL10 cytokine family member IL-20. Furthermore, the IL20 promoter is controlled by CpG DNA methylation. Inflammasomes are multi-protein complexes that become activated in response to “danger signals.” Furthermore, the expression of inflammasome components (NLRP3 and ASC/PYCARD) is regulated by epigenetic events. Promotors of NLRP3 and PYCARD are controlled by CpG DNA methylation. (B) In monocytes from CNO/CRMO patients, ERK1/2 activation in response to LPS stimulation is impaired which results in reduced Sp-1 activation and nuclear shuttling, and decreased H3S10 phosphorylation at IL10 and IL19 resulting in impaired gene expression. Reduced levels of immune regulatory cytokine expression allows for higher expression of the pro-inflammatory cytokine IL-20. Furthermore, impaired IL-10 and IL-19 expression promoted expression of inflammasome components, and reduced CpG DNA methylation of the PYCARD and NLRP3 genes further increase pro-inflammatory molecule expression. Thus, epigenetic events are involved in the molecular pathophysiology of CNO/CRMO.