Figure 8.
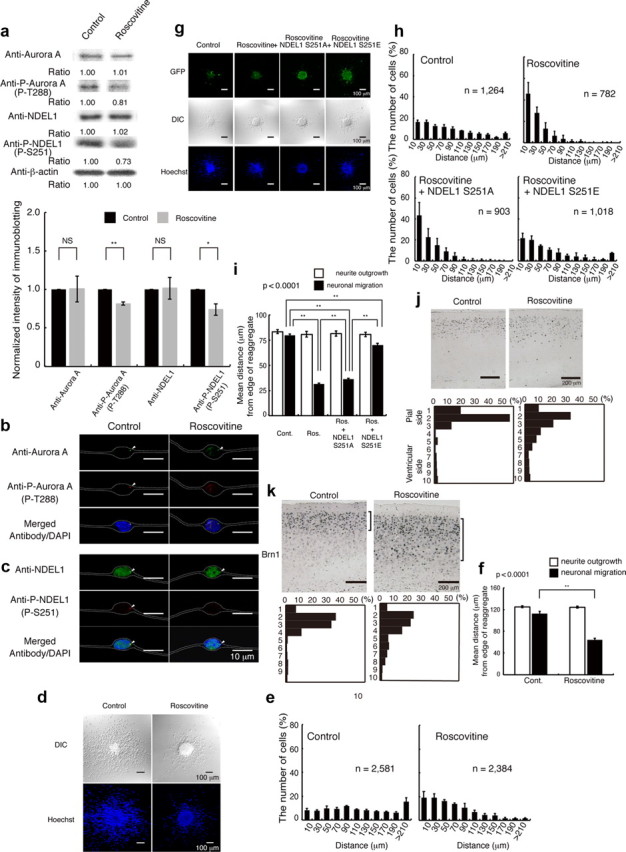
Inhibition of CDK5 by roscovitine resulted in defective neuronal migration, which was rescued by expression of GFP-Ndel1 (S251E). a, Granular neurons were treated with roscovitine. Western blotting was performed 24 h after start of culture. Aurora-A and NDEL1 displayed similar expression levels with untreated neurons, whereas the levels of phosphorylated Aurora-A and NDEL1 proteins were decreased after treatment with roscovitine. Relative intensities of the bands of Western blotting are shown at the bottom. Intensity was normalized to β-actin. Statistical examination was performed by unpaired Student's t test, with *p < 0.05 and **p < 0.01 (bottom panel). b, Immunocytochemistry against either Aurora-A (b) or NDEL1 (c) revealed similar expression levels after treatment, whereas phosphorylated proteins were decreased after treatment with roscovitine. The white dotted lines indicate the outline of granular neurons. d, Migration assay using cerebellar granule neurons. DIC images (top) and Hoechst staining images (bottom) are shown. e, The migration distance of each neuron 24 h after the start of culture was binned. n is the number of neurons measured for each examination. Wild-type neurons displayed normal migration distances, whereas neurons treated with roscovitine displayed a shift in the distribution of bins toward the left. f, Mean length of neurites (open bar) and nuclear position (solid bar) from the edge of central aggregation. Statistical examination was performed by an ANOVA followed by t test with correction: **p < 0.01. g, Rescue experiments after roscovitine treatment with control GFP, GFP-Ndel1 (S251A), or GFP-Ndel1 (S251E) transfection. GFP images are shown. Expression of GFP-Ndel1 (S251A) had no effect on defective migration, whereas expression of GFP-Ndel1 (S251E) significantly improved migration after roscovitine treatment. h, The migration distance of each neuron 24 h after the start of culture was binned. n is the number of neurons measured for each examination. Expression of GFP-Ndel1 (S251E) significantly improved migration, which was characterized by a rightward shift. i, Mean length of neurites (open bar) and nuclear position (solid bar) from the edge of central aggregation. Statistical examination was performed by an ANOVA followed by t test with correction: **p < 0.01. j, BrdU birth-dating analysis revealed neuronal migration defects after intraperitoneal injection of roscovitine. Mice were injected with BrdU at E14.5 and killed at P21. Roscovitine was injected twice at E15.5 and E17.5. Quantitative analysis was performed by measuring the distribution of BrdU-labeled cells in 10 bins that equally divided the cortex from ML to SP (bottom panels). The staining patterns are representative of 10 different experiments. Note the shift downward of neuron localization toward the ventricular side after intraperitoneal injection of roscovitine. k, The distribution of Brn-1-positive cells is indicated at the right side of each panel. Brn-1-positive cells were more dispersed after intraperitoneal injection of roscovitine. Quantitative analysis was performed by measuring the distribution of Brn-1-positive cells in each of 10 bins that equally divided the cortex from ML to SP (bottom panels). The staining patterns are representative of 10 different experiments.
