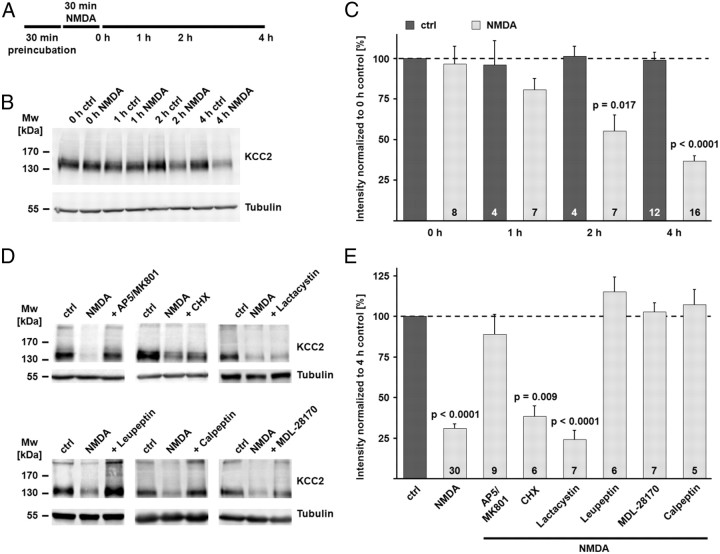
Figure 4.
NMDA-induced excitotoxicity leads to calpain-mediated KCC2 degradation. A, Scheme of the experimental design. Coronal brain slices were preincubated in the presence of different combinations of inhibitors (see Materials and Methods for details). At the end of the preincubation, NMDA (100 μm) was added for 30 min. The end of the NMDA incubation was defined as time point 0 h. B, The NMDA incubation induced a rapid decrease in the KCC2 protein level. Tubulin was used as a loading control. C, The mean values of the quantified immunoblot signals showed a significant decrease in the KCC2 signal after the NMDA incubation (80.6 ± 7.0% of the KCC2 protein level in the 0 h control after 1 h; 55.1 ± 9.8% after 2 h; 36.6 ± 3.4% after 4 h), while the signal remained stable under control conditions (98.7 ± 5.0% after 4 h). D, E, Application of NMDA in the presence of the NMDAR antagonists AP5 and MK-801 (100 and 10 μm) blocked the degradation of KCC2 (89.0 ± 12.1%). The protein synthesis inhibitor cycloheximide had no effect on the NMDA-induced degradation of KCC2 (38.5 ± 6.3%). The proteasome inhibitor lactacystin (1 μm) did not prevent the downregulation of KCC2 (24.3 ± 5.7%). The inhibitor of lysosomal degradation, leupeptin (50 μm), blocked the decrease in KCC2 protein (115.3 ± 8.8%). The two calpain inhibitors MDL-28170 (MDL) (30 μm) and calpeptin (30 μm) both blocked the NMDA-induced degradation of KCC2 (102.9 ± 5.7% and 107.3 ± 9.2%). Statistical significance was assessed by Kruskal–Wallis one-way ANOVA on Ranks with Mann–Whitney's post hoc test. The values for n are given in the bar diagram. Error bars denote SEM.