Figure 4.
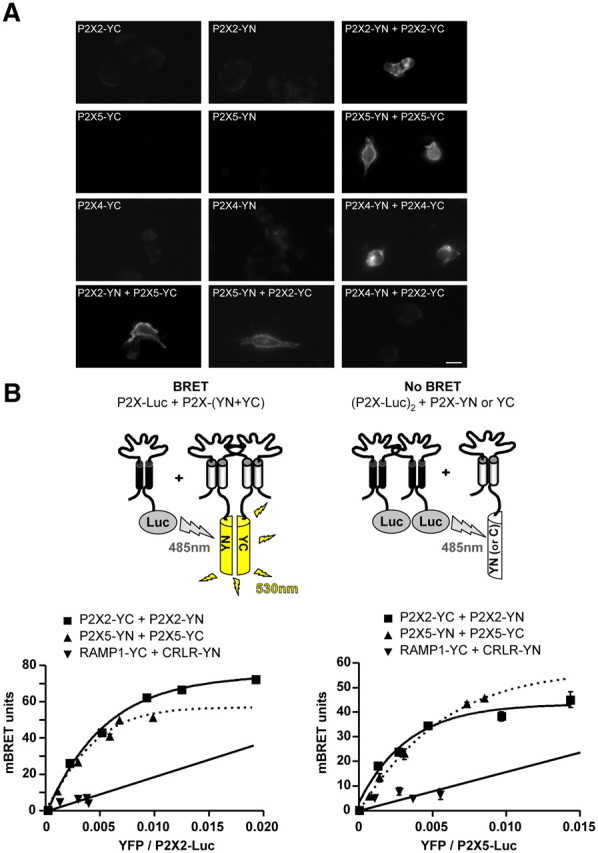
Stoichiometry of P2X2 and P2X5 interactions as assessed by BiFC and BRET/BiFC. A, Bimolecular fluorescence complementation between P2X subunits. P2X subunits fused to either the amino or carboxyl half of YFP (YN or YC) were transfected alone or in combination in HEK cells. Recomplemented fluorescence was observed by microscopy. BiFC was observed between homomeric P2X2, P2X4, and P2X5 subunits, as well as between heteromeric P2X2 and P2X5 subunits, but not between P2X2 and P2X4 subunits. Scale bar, 10 μm. B, Combination of BRET and BiFC reveals the stoichiometry of the heteromeric P2X2/P2X5 assembly. Top, Diagram illustrating the approach. BRET is only observed between one P2X2-Luc subunit and any other combinations of two P2X-hemi-YFP subunits. BRET titration curves between P2X2-Luc (left) or P2X5-Luc (right) cotransfected with P2X2, P2X5, RAMP1, and CRL fused to hemi-YFP. Specific BRET signals were observed between P2X2 and P2X5 subunits for each experimental condition, demonstrating the existence of heteromeric receptors with two different stoichiometries. Data are expressed as mean ± SEM of at least N = 3 experiments.
