Figure 13.
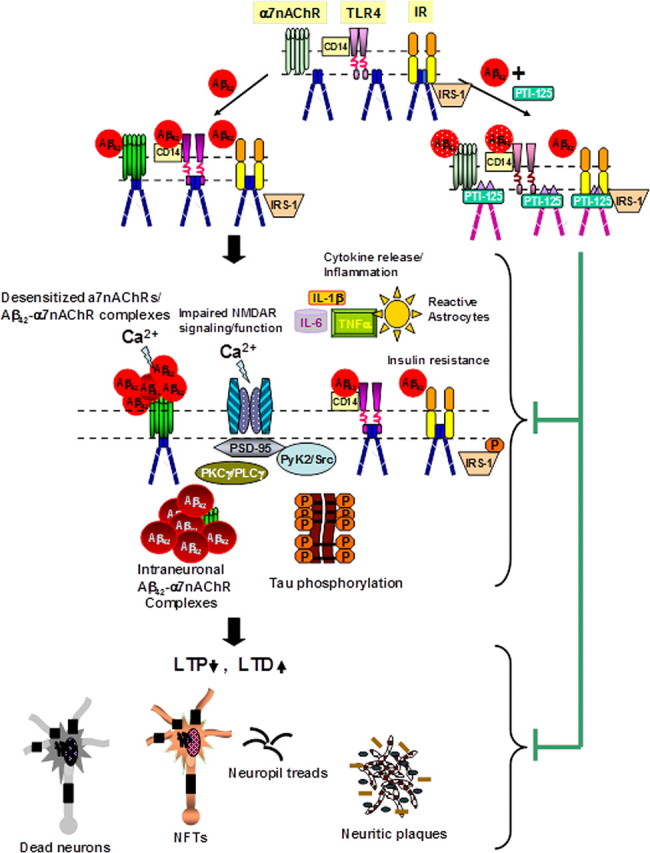
Proposed model of PTI-125 normalizing Aβ42-induced synaptic dysfunction. Soluble Aβ42 or Aβ42 oligomers bind to α7nAChRs in AD brains to induce synaptic dysfunction by first activating α7nAChRs (Dougherty et al., 2003) and TLR4s (Lotz et al., 2005) (by CD14) to recruit FLNA, which enables a high-affinity interaction of Aβ42 with these receptors. The result of FLNA recruitment to TLR4 is enhanced signaling and cytokine release. With FLNA recruited to α7nAChR, Aβ42 binding activates kinases that leads to rapid tau phosphorylation and neurofibrillary lesions (Wang et al., 2003) and causes desensitization, restricting Ca2+ influx through α7nAChRs and impairing downstream NMDAR activity and signaling. In addition, Aβ42 oligomers acting through multiple pathways impair IR function, leading to insulin resistance in neuronal cells (Zhao et al., 2008). Similar to a partial pharmacological blockade of NMDARs, Aβ42-induced hypofunctioning NMDARs are expected to reduce NMDAR-dependent LTP and to increase LTD, leading to dendritic spine shrinkage and retraction (Shankar et al., 2007) that further dampens excitatory neurotransmission. By binding to a defined pentapeptide binding domain with high affinity, PTI-125 may elicit conformational changes in FLNA to prevent its recruitment to α7nAChRs and TLR4s, thereby reducing Aβ42 affinity and facilitating the dissociation of Aβ42 from these receptors. The normalized α7nAChR and TLR4 activities improve NMDAR activation and reduce cytokine release (inflammation). Although neither Aβ42 nor PTI-125 alters FLNA recruitment to IRs, the PTI-125-induced conformational change in FLNA restores Aβ42-suppressed IR function and lessens insulin resistance. The augmented NMDAR activation favors LTP induction and healthier dendritic spines, resulting in more normal excitatory neurotransmission and cognitive processing. The improved IR function leads to healthier cells. Together, PTI-125 treatment lessens neurodegeneration and reduces the burdens of neuritic plaques and neurofibrillary tangles. Changes in color and shape of receptors and FLNA represent conformational or affinity changes.
