Figure 2.
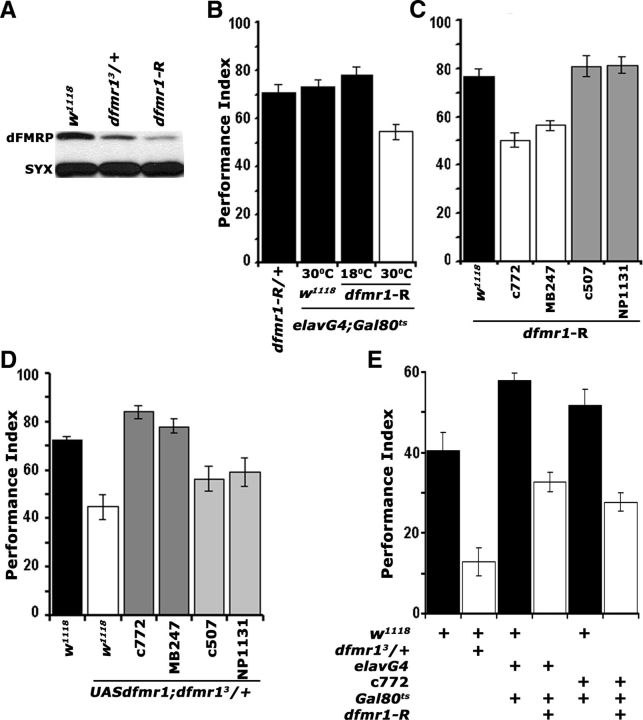
dFMRP is required within the mushroom bodies for associative learning and memory. A, Western blot demonstrating the levels of dFMRP in adult head lysates of w1118 controls, dfmr13/+ heterozygotes, and animals expressing pan-neuronally the interfering UAS-dfmr1-R transgene. This represents one of four identical blots with similar results. dFMRP was revealed with the α-dfmrP 5A11 antibody, and Syntaxin (SYX) was used as loading control. B, Adult-specific pan-neuronal expression of UAS-dfmr1-R (30°C) yields significant (p < 0.0001, Dunnett's) learning deficits (open bars) compared with uninduced (18°C), transgene-alone and driver-alone (black bars) controls. n ≥ 8. All animals were heterozygous for the transgene Gal4 and Gal80ts. All animals were trained and tested at 25°C, regardless of the temperatures they were maintained before training to manipulate transgene expression as indicated. C, Spatially restricted abrogation of dFMRP. All animals were heterozygous for UAS-dfmr1-R and Gal4 drivers. (ANOVA, p < 0.0001). Attenuation specifically in α/β and γ MB lobes with c772 and MB247 (white bars) impaired learning (p < 0.0001 vs w1118/UAS-dfmr1-R controls; black bars) and phenocopies the dfmr13/+ mutant phenotype. In contrast, restricted dfmr1-R expression only within the γ lobes with NP1131, or to the ellipsoid body with c507 did not affect learning (gray bars; p = 0.023 and p = 0.367, respectively). n ≥ 10. D, A UAS-dfmr1 cDNA transgene recombined onto the chromosome harboring the dfmr13 mutation, fully rescues (darker gray bars) the dfmr13/+ learning deficit (white bars) when expressed in the MBs with c772 (p = 0.135 vs w1118; black bar) and MB247 (p = 0.667 vs w1118; black bar), but not when expressed γ lobes with NP1131, or to the ellipsoid body with c507 (lighter gray bars; p < 0.0001). All animals were heterozygous for the transgene and Gal4 drivers. n ≥ 12. E, Twenty-four hour memory induced with five rounds of spaced training was significantly impaired in dfmr13 heterozygotes (open bars) and animals with adult-specific, pan-neuronal, or MB-specific dfmr1-R expression (p < 0.0001 for all pairwise comparisons with their specific yoked controls; black bars). n ≥ 12. All animals were heterozygous for the transgene Gal4 and Gal80ts and were trained and tested at 25°C, regardless of the temperatures they were maintained before training to manipulate transgene expression as indicated. Error bars indicate SEM.