Figure 5.
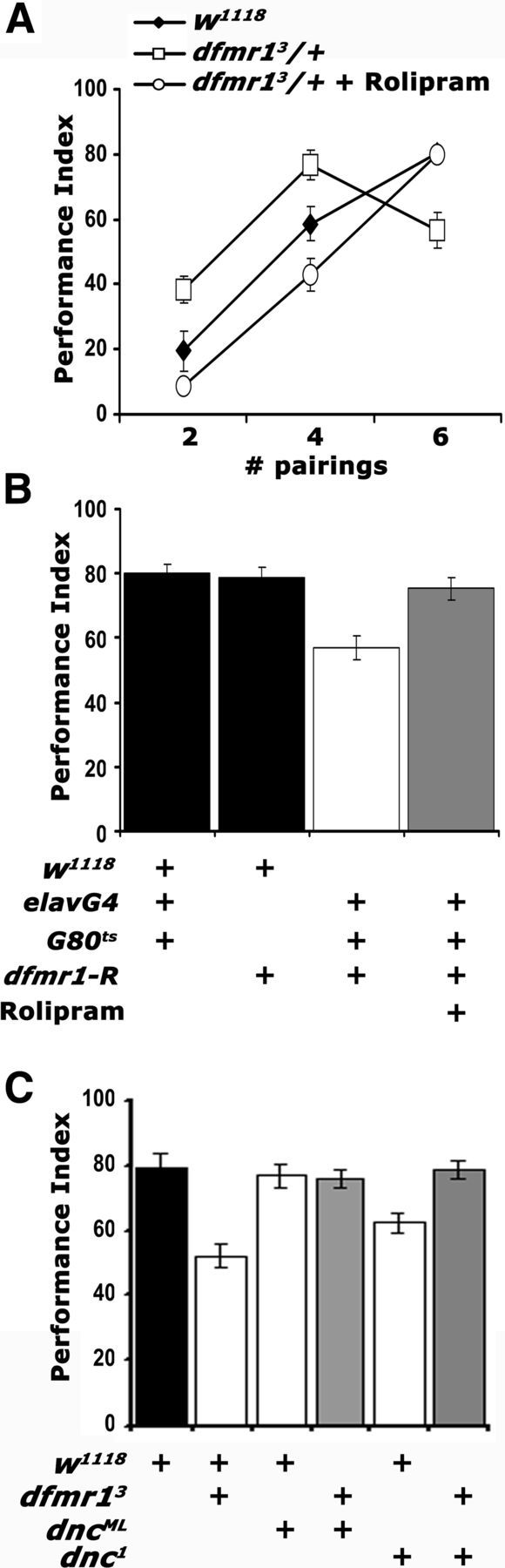
cAMP elevation in dfmr13/+ reverses their learning deficit. Mean PIs and their SEMs (PI ± SEM) are shown for all experiments. Where appropriate, animals were heterozygous for all transgenes, Gal4 drivers, and Gal80ts. A, Rolipram administration (open circles) resulted in performance indistinguishable from that of controls (filled diamonds) after two, four, and six pairing training (p = 0.16, p = 0.04, and p = 0.73, respectively). The performance of untreated animals (open squares) remained significantly different from both (p < 0.001). n ≥ 8. B, Rolipram administration (gray bar) to animals with pan-neuronally abrogated dfmr1 resulted in learning equivalent to that of control driver heterozygotes (p = 0.44) or UAS-dfmr1-R heterozygotes (p = 0.98). The performance of untreated animals (open bars) remained significantly different from all other groups (p < 0.0001). n ≥ 10. C, Genetic rescue of the dfmr13/+ learning deficit by reduction of functional DNC. The performances of dnc1/+; dfmr13/+ double heterozygotes and dncML/+; dfmr13/+ (gray bars) are not statistically distinguishable (p = 0.15 and p = 0.36, respectively) from that of w1118 controls (black bar). In contrast, the performances of dnc1 heterozygotes and dfmr13/+ (open bars) were significantly different from controls (p < 0.0001 for both). n ≥ 8.
