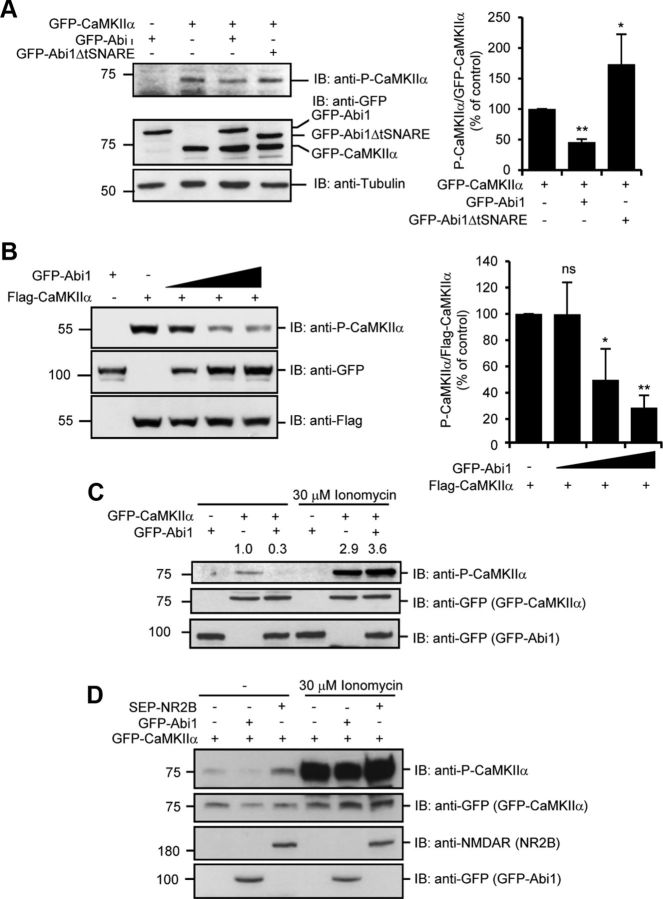
Figure 3.
Binding of Abi1 inhibits P-CaMKIIα. A, Levels of CaMKIIα Thr 286 autophosphorylation were examined by immunoblotting (IB) with anti-phospho-Thr 286 CaMKIIα antibodies (P-CaMKIIα) in HeLa cells transfected with indicated constructs. Results from three independent experiments were quantified, normalized to GFP-CaMKIIα, and expressed as percentage of the amount in control cells (mean ± SD, *p = 0.003, **p = 0.00003, Student's t test). B, HeLa cells were transfected with the increasing levels of GFP-Abi1. Anti-Flag blot was used as the loading control. Results from three independent experiments were quantified, normalized to Flag-CaMKIIα, and expressed as the percentage of the amount in control cells (mean ± SD; ns, not significant; *p = 0.05, **p = 0.004, Student's t test). C, HeLa cells transfected with GFP-CaMKIIα and GFP-Abi1 were treated with or without 30 μm ionomycin and 2 mm CaCl2 for 10 min and examined by immunoblotting with indicated antibodies. Changes in P-CaMKIIα were quantified and expressed in arbitrary units as changes in P-CaMKIIα/GFP-CaMKIIα compared with control cells. D, HeLa cells were transfected with GFP-CaMKIIα with or without GFP-Abi1 or SEP-NR2B. Cells were treated with or without 30 μm ionomycin for 10 min and immunoblotted with indicated antibodies.