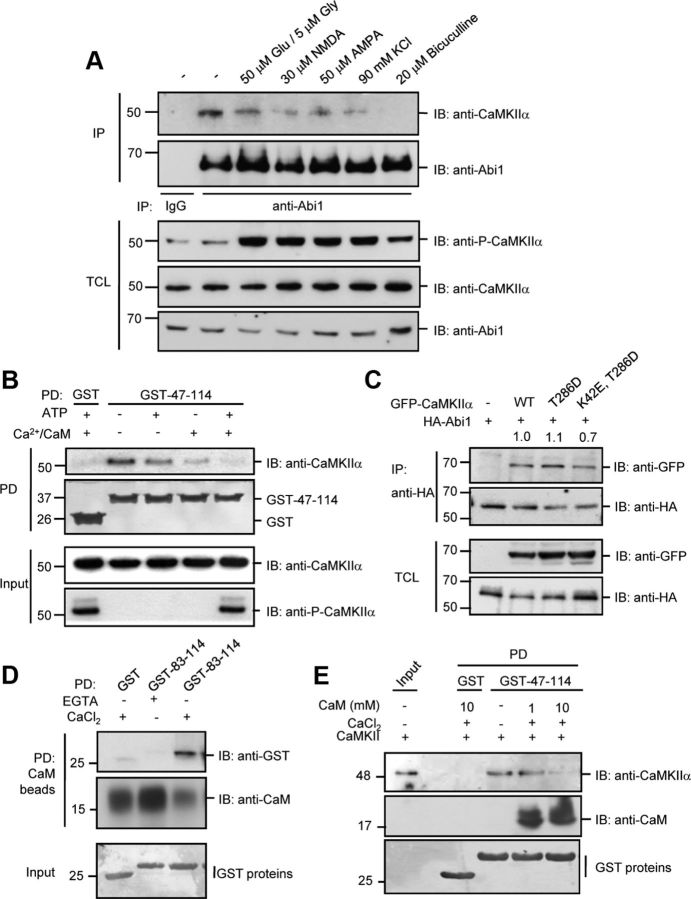
Figure 5.
Interaction between Abi1 and CaMKIIα is disrupted by excitatory stimulation through Ca2+/CaM binding to Abi1. A, 14 DIV rat cortical neurons were treated with 50 μm glutamate and 5 μm glycine for 5 min, 30 μm NMDA for 5 min, 50 μm AMPA for 5 min, 90 mm KCl for 3 min, or 20 μm bicuculline for 30 min. Lysates were immunoprecipitated (IP) with anti-Abi1 antibodies, subjected to SDS-PAGE, and then immunoblotted (IB) with anti-CaMKIIα antibodies. B, Purified CaMKII was incubated with ATP and/or 0.3 mm Ca2+/ 2 mm CaM at 30°C for 10 min, to promote activation of CaMKII, and then the purified GST or GST-Abi1 tSNARE (GST-47–114) protein was added to the mixture and GST pull-down (PD) assays were performed. The interaction of Abi1 tSNARE with CaMKII was analyzed by immunoblotting with anti-CaMKIIα antibodies. C, HeLa cells were cotransfected with HA-Abi1 and GFP-CaMKIIα constructs (wild-type, autophosphorylation mimetic GFP-CaMKIIαT286D, or autophosphorylation mimetic kinase-dead GFP-CaMKIIαK42E,T286D), and the lysates were immunoprecipitated with anti-HA antibodies and immunoblotted with anti-GFP antibodies. Ratio of bound GFP-CaMKIIα mutants to HA-Abi1 were quantified and expressed as arbitrary units compared with wild type. D, CaM Sepharose beads were used for pull-down of GST-83-114 in the presence of 1 mm EGTA or 2 mm CaCl2. Pull-down assays were analyzed by Western blot as indicated. E, 1 or 10 mm CaM, and 0.3 mm CaCl2 were incubated with purified CaMKII and GST-47-114. Binding of CaMKII and CaM to GST-47-114 was observed by immunoblotting with indicated antibodies. TCL, Total cell lysate.