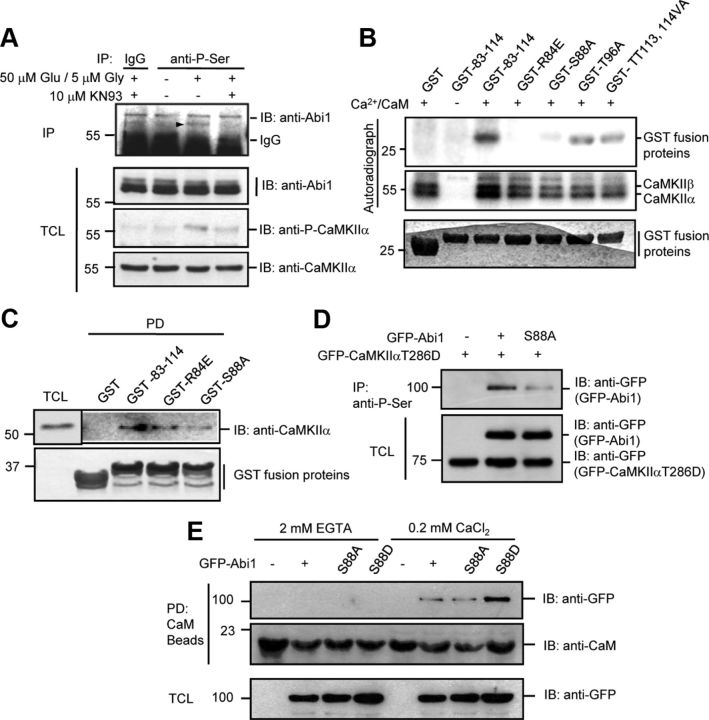
Figure 6.
Abi1 is phosphorylated at serine 88 by CaMKIIα in vitro and in vivo and this phosphorylation enhances Abi1 binding to CaM. A, We treated 14 DIV cortical neurons with 50 μm glutamate and 5 μm glycine for 5 min with or without the pretreatment of 10 μm KN93 for 30 min. The lysates were immunoprecipitated (IP) with antiphosphoserine antibodies (anti-P-Ser) and immunoblotted (IB) with anti-Abi1 antibodies. Immunoprecipitated Abi1 is indicated with an arrowhead. B, In vitro, serine 88 in Abi1 tSNARE domain is a major phosphorylation site by CaMKII. Before pull-down (PD) assays, CaMKII was incubated with γ-P32-ATP and/or Ca+2/CaM at 30°C for 10 min to initiate CaMKII activation, and then aliquoted to samples containing wild-type (GST-83–114) or mutant GST-tSNARE proteins (GST-R84E, GST-S88A, and GST-TT113,114VA) and incubated at 30°C for 30 min. Phosphorylation of the GST fusion proteins and CaMKII was observed by autoradiography. C, Purified CaMKII was incubated with the wild-type and the mutant GST-tSNARE proteins and GST pull-down precipitates were analyzed by Western blot. D, Lysates of HeLa cells cotransfected with constitutively active GFP-CaMKIIαT286D and wild-type GFP-Abi1 or GFP-Abi1S88A were immunoprecipitated with anti-P-Ser antibodies and immunoblotted with anti-GFP antibodies. E, HeLa cells transfected with GFP-Abi1, GFP-Abi1S88A, or GFP-Abi1S88D were incubated with CaM Sepharose beads in the presence of 2 mm EGTA or 0.2 mm CaCl2. Pull-down precipitates were subjected to SDS-PAGE and immunoblot with indicated antibodies. TCL, Total cell lysate.