Abstract
Background
Results of the landmark Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) trial comparing rhythm control and rate control strategies has led to dramatic changes in the pharmacological management of non-valvular atrial fibrillation (NVAF) patients. We sought to investigate the effect of antiarrhythmic drugs (AADs) on the clinical outcomes of NVAF patients using “real-world” data from China.
Material/Methods
We evaluated the association between AAD usage and clinical outcomes using clinical data of 8161 NVAF patients who were AAD-naive before enrollment in the China Atrial Fibrillation Registry, recruited between August 2011 and February 2017. The primary outcome was all-cause mortality.
Results
Compared with 6167 patients who never used any AADs, 1994 patients in the AAD group had lower incidence (per 100 person-years) of all-cause mortality (1.44 versus 3.91), cardiovascular death (0.45 versus 2.31), ischemic stroke (1.36 versus 2.03), and cardiovascular hospitalization (9.83 versus 10.22) over a mean follow-up duration of 316.7±90.4 days. After adjusting for potential confounders, AAD usage was associated with a lower risk of all-cause mortality [hazard ratio (HR): 0.50, 95% confidence interval (CI): 0.31–0.81] and decreased risk of cardiovascular death (HR: 0.30, 95% CI: 0.13–0.68). Subgroup analysis revealed AAD was associated with higher risk of cardiovascular hospitalization among female patients.
Conclusions
AAD usage was associated with lower risk of 1-year all-cause mortality and cardiovascular death in “real-world” patients with NVAF.
MeSH Keywords: Anti-Arrhythmia Agents, Atrial Fibrillation, Prognosis
Background
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, carrying a substantially increased risk of morbidity and mortality. AF negatively impacts quality of life of patients and imposes significant burden on health care systems around the world [1–4]. Antiarrhythmic drugs (AADs) are traditionally regarded as a cornerstone for AF management to restore and maintain sinus rhythm. However, the landmark Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study [5] and other subsequent randomized controlled trials [6–12] reported no survival advantage of rhythm-control strategy over rate-control drugs. Contrastingly, recent systematic reviews suggested rhythm control may be beneficial for a subgroup of younger and healthier patients [13,14]. Moreover, registry studies also demonstrated insignificant [15,16] or significant [17] improvements in overall mortality for rhythm control strategy in comparison to rate control strategy. Due to uncertainties relating to the safety and efficacy of AADs, clinicians may avoid prescribing AAD for high risk patients according to the AF guidelines [3,4,18] or use medicines like sotalol [19,20], which have reported adverse survival effect, in their practice. An “up-to-date” investigation of the effects of AAD on clinical outcomes of AF patients using current “real-world” data is important in the context of significant changes in the selection of patients and AAD agents. As such, our study aimed to evaluate the association between AAD usage and patient prognosis using data from the China Atrial Fibrillation registry (China-AF) study.
Material and Methods
Study population
The rationale and design of the China-AF study have been described previously [21,22]. In brief, the China-AF study is a prospective, multicenter, hospital-based ongoing registry study. Between August 2011 and February 2017, 20 666 patients were enrolled from 31 tertiary and non-tertiary hospitals located in Beijing. The Human Research Ethics Committee at Beijing Anzhen Hospital approved this study and the ethics review boards at individual participating hospitals approved their participation. Written informed consent was obtained from each patient.
Patients with non-valvular atrial fibrillation (NVAF) were identified from the China-AF study database. For the present analyses, we excluded patients less than 18 years of age (n=1), those with valvular AF (n=579), those who had less than 6 months’ follow-up (n=634) or lacked follow-up data (n=757), those who had prior AAD usage before registry enrollment (n=7340), and those with catheter ablation or surgical ablation (n=3078) during the index hospitalization. Patients who received ablation therapy during follow-up were censored at the time of ablation except for the 116 excluded patients as the duration period between their registry enrollment and ablation was less than 6 months.
There were 1994 patients who received either class I (propafenone, moricizine, mexiletine) or class III (amiodarone, sotalol) AAD upon registry enrollment and during follow-up and who were classified into the AAD group, and were censored at the time of discontinuation of AAD usage during the follow-up period according to the “as treated” definition of exposure. Within the AAD group, 689 patients (34.5%) received amiodarone therapy. Propafenone and sotalol were used in 458 patients (23.0%) and 93 patients (4.7%) respectively. The remaining 754 patients (37.8%) received other antiarrhythmic agents (such as moricizine), switched between different AADs, or had a combination of AADs. Patients who did not use any of the aforementioned AADs were classified into the non-AAD group (Figure 1), and they were censored at 1-year after registry enrollment.
Figure 1.
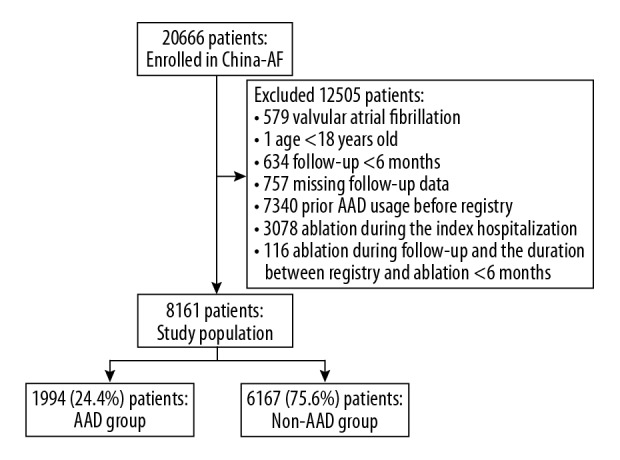
Patient flowchart.
Data collection
Upon patient enrollment, data on sociodemographic characteristics (age, gender, education status, and medical insurance coverage), medical history including established coronary artery disease (CAD), diabetes mellitus, hypertension, hyperlipidemia, chronic heart failure (CHF), major bleeding, previous stroke/transient ischemic attack (TIA)/peripheral thromboembolism (TE), liver function, renal function (presented as estimated glomerular filtration rate, eGFR), AF type (new-onset, paroxysmal or persistent) and time of AF diagnosis, medication history, as well as the patient treatment site were collected. We used multiple imputation to fill in the missing values. Patients were followed up at 3 months, 6 months, and every 6 months thereafter by trained staff at the outpatient clinics or through telephone interview. Data on heart rhythm, medical therapies, cardioversion, and outcomes including all-cause death, cardiovascular death, nonfatal strokes, and hospitalizations were recorded.
Established CAD was defined as having any history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting. Abnormal liver function was defined as having serum level of aspartate aminotransferase or alanine aminotransferase >120 U/L and total bilirubin >34.2 μmol/L. Estimated glomerular filtration rate (eGFR) was calculated using the abbreviated equation from the Modification of Diet in Renal Disease study [23]. Definitions of ischemic stroke and cardiovascular hospitalization (CVH) were adopted from the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards report [24].
Study outcomes
The primary outcome of the study was all-cause mortality and the secondary outcomes were cardiovascular death, ischemic stroke, and CVH separately. Outcomes occurring prior to AAD usage were not considered events of interest. We also evaluated the rate of sinus rhythm maintenance at the penultimate follow-up.
Statistical analysis
Descriptive data were presented as mean ± standard deviation (SD) for continuous data or number (percentage) for categorical data. Baseline characteristics and clinical outcomes were compared between AAD group and non-AAD group using the Student’s t-test (for continuous variables) or the chi-square test (for categorical variables).
Rates of primary and secondary outcomes occurrence during follow-up were depicted in Kaplan-Meier plots and compared using the log-rank test. Cox proportional hazards regression model were used to evaluate the hazard ratios (HRs) and their 95% confidence intervals (CIs) of AAD usage with each outcome. Before modeling, we removed the survival person-time between registry entry and the first prescription of AAD during follow-up to minimize the immortal time bias [25]. Multivariate models were adjusted for potential confounders including baseline age, sex, education status (high school completion), health insurance coverage (partial or complete health insurance coverage), body mass index (BMI), smoking and drinking status (current smoking and current drinking), history of established CAD, diabetes mellitus, hypertension, hyperlipidemia, CHF, previous bleeding, stroke/TIA/TE, abnormal liver function, eGFR <60 mL/min/1.73 m2, AF type (persistent AF) and diagnosis of AF ≥12 months, and hospital level (tertiary hospital). We also included oral anticoagulant (OAC) usage and hospitalization history at the penultimate follow-up as time-dependent covariates in the multivariable models. Subgroups analysis was conducted to explore the differential effects of AAD use on the risk of clinical outcomes by age (<75 years versus ≥75 years), sex, previous CAD, CHF, AF type (paroxysmal versus persistent) and time since AF diagnosis (<12 versus ≥12 months). Rate of sinus rhythm maintenance was evaluated by chi-square test. For the sensitivity analyses, we prolonged observation period up to 2 years after registry enrollment.
All statistical tests were 2-tailed and P value <0.05 was considered statistically significant. All analyses were conducted by an external contract research organization, using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
Among 8161 patients with NVAF included in the present study, 1994 individuals (24.4%) received AADs therapy. Table 1 shows that patients treated with AAD were younger (mean age 66.9 versus 68.6 years old), more frequently completed high school education, had fewer comorbidities such as CAD, diabetes mellitus, hypertension, CHF, prior bleeding and stroke, and were more likely to be treated in tertiary hospitals. The proportion of persistent AF was much lower among users of AADs, who were also less likely to be taking concomitant OAC. The β-blockers usage in the AAD group and non-AAD group was 51.9% and 57.2%, and the usage of digoxin was 7.2% and 14.1% in each group, respectively (Table 1). Cardioversion was conducted in 1.7% of AAD group patients and in 0.8% of non-AAD group patients. During follow-up, cardioversion was applied in 0.2% of all patients.
Table 1.
Baseline patient characteristics by antiarrhythmic drug usage.
| Patient characteristics* | Overall (N=8161) | AAD group (N=1994) | non-AAD group (N=6167) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD), years | 68.2 (11.8) | 66.9 (11.5) | 68.6 (11.9) | <0.001 |
| Male | 4737 (58.0) | 1112 (55.8) | 3625 (58.8) | 0.018 |
| High school completion | 2054 (28.0) | 596 (33.4) | 1458 (26.3) | <0.001 |
| Partial or complete health insurance coverage | 7524 (92.3) | 1839 (92.3) | 5685 (92.2) | 0.967 |
| BMI, mean (SD), kg/m2 | 25.4 (3.7) | 25.4 (3.7) | 25.4 (3.7) | 0.962 |
| Current smoking | 1253 (15.5) | 296 (14.9) | 957 (15.7) | 0.414 |
| Current drinking | 1493 (18.5) | 354 (17.9) | 1139 (18.7) | 0.428 |
| Medical history | ||||
| Established CAD** | 1320 (16.2) | 294 (14.8) | 1026 (16.7) | 0.045 |
| DM | 2255 (27.6) | 496 (24.9) | 1759 (28.5) | 0.002 |
| Hypertension | 5673 (69.5) | 1347 (67.6) | 4326 (70.2) | 0.026 |
| Hyperlipidemia | 3591 (44.0) | 891 (44.7) | 2700 (43.8) | 0.505 |
| CHF | 1898 (23.3) | 296 (14.8) | 1602 (26.0) | <0.001 |
| Previous bleeding | 412 (5.1) | 71 (3.6) | 341 (5.5) | 0.001 |
| Previous stroke/TIA/TE | 1610 (19.7) | 302 (15.2) | 1308 (21.2) | <0.001 |
| Abnormal liver function# | 260 (4.5) | 50 (3.7) | 210 (4.8) | 0.092 |
| OAC usage | 1652 (20.3) | 280 (14.1) | 1372 (22.3) | <0.001 |
| eGFR, mean (SD), mL/min/1.73 m2## | 102.7 (32.8) | 105.1 (31.2) | 102.0 (33.2) | 0.003 |
| AF type | ||||
| New-onset AF | 944 (11.6) | 263 (13.2) | 681 (11.1) | 0.010 |
| Paroxysmal AF | 3547 (43.5) | 1157 (58.1) | 2390 (38.8) | <0.001 |
| Persistent AF | 3655 (44.9) | 573 (28.8) | 3082 (50.1) | <0.001 |
| Diagnosis of AF ≥12 months | 4284 (52.5) | 1008 (50.6) | 3276 (53.1) | 0.046 |
| Rate-lowering drugs | ||||
| β blockers | 4561 (55.9) | 1035 (51.9) | 3526 (57.2) | <0.001 |
| Non-dihydropyridine Calcium-channel antagonists | 553 (6.8) | 139 (7.0) | 414 (6.7) | 0.691 |
| Digoxin | 1014 (12.4) | 143 (7.2) | 871 (14.1) | <0.001 |
| Tertiary hospital admission | 6381 (78.2) | 1632 (81.9) | 4749 (77.0) | <0.001 |
| Inpatients | 3078 (37.7) | 697 (35.0) | 2381 (38.7) | 0.003 |
| Follow-up duration, mean (SD), d | 316.7 (90.4) | 242.0 (112.5) | 340.8 (65.8) | <0.001 |
AAD – antiarrhythmic drug; AF – atrial fibrillation; BMI – body mass index; CAD – coronary artery disease; CHF – chronic heart failure; DM – diabetes mellitus; eGFR – estimated glomerular filtration rate; OAC – oral anticoagulants; SD – standard deviation; TE – thromboembolism; TIA – transient ischemic attack.
Continuous variables were presented as mean (SD) and categorical variables were presented as number (percent);
Established CAD includes myocardial infarction, percutaneous coronary intervention and coronary artery bypass grafting;
Liver function was obtained in 5780 patients (1362 in the AAD group and 4418 in the non-AAD group).
Abnormal liver function was defined as serum level of aspartate aminotransferase or alanine aminotransferase >120 U/L, and total bilirubin >34.2 μmol/L;
eGFR was obtained in 5731 patients (1342 in the AAD group and 4389 in the non-AAD group).
eGFR (mL/min/1.73 m2)=186×(SCr [μmol/L]×0.0113)−1.154×age−0.203×0.742 (if female).
Clinical outcomes
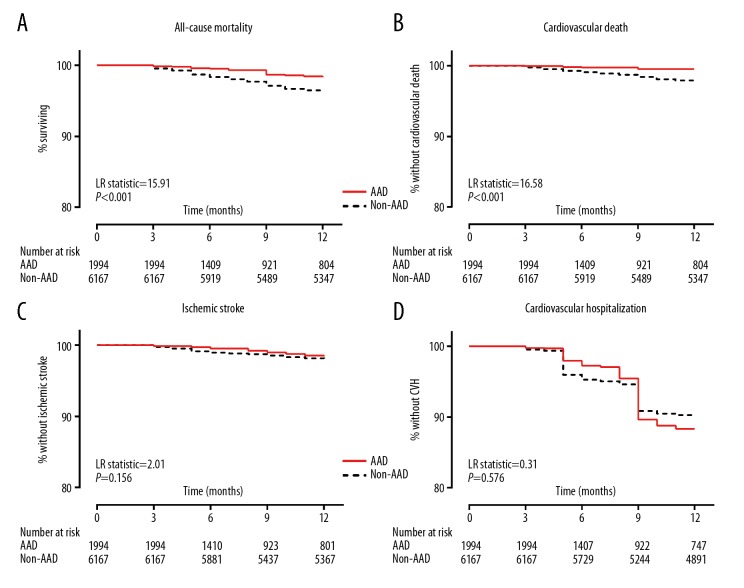
The event-free-survival curves are shown in Figure 2. Compared with the non-AAD group, patients in the AAD group had lower incidence (per 100 person-years) of all-cause mortality (1.44 versus 3.91), cardiovascular death (0.45 versus 2.31), ischemic stroke (1.36 versus 2.03), and CVH (9.83 versus 10.22) over a mean follow-up duration of 316.7±90.4 days. After adjusting for potential baseline confounders and time-dependent covariates including OAC usage and treatment site at the penultimate follow-up, the AAD group patients were at significantly lower risk of all-cause mortality and lower risk of cardiovascular mortality [adjusted HR: 0.50 (0.31–0.81) and adjusted HR: 0.30 (0.13–0.68), respectively, Table 2]. There was no relationship between AAD usage and risk of ischemic stroke and CVH (both P value ≥0.176; Table 2, Supplementary Table 1).
Figure 2.
Kaplan-Meier curves for 1-year clinical outcomes in (NVAF). This figure shows Kaplan-Meier curves for all-cause mortality (A), cardiovascular death (B), ischemic stroke (C) and (CVH) (D) among patients with NVAF enrolled in China Atrial Fibrillation Registry between 2008 and 2015 by AAD usage status. NVAF – non-valvular atrial fibrillation; CVH – cardiovascular hospitalization; AAD – antiarrhythmic drugs.
Table 2.
Association between AAD usage and patient outcomes at 1 year.
| AAD (N=1994) | Non-AAD (N=6167) | Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|---|---|
| Events | Rate** | Events | Rate | HR (95%CI)# | P Value | HR (95%CI) | P Value | |
| All-cause mortality | 19 | 1.44 | 225 | 3.91 | 0.41 (0.26–0.66) | <0.001 | 0.50 (0.31–0.81) | 0.005 |
| Cardiovascular death | 6 | 0.45 | 133 | 2.31 | 0.22 (0.10–0.50) | <0.001 | 0.30 (0.13–0.68) | 0.004 |
| Ischemic stroke | 18 | 1.36 | 116 | 2.03 | 0.73 (0.44–1.20) | 0.207 | 0.70 (0.42–1.17) | 0.176 |
| CVH | 129 | 9.83 | 570 | 10.22 | 1.09 (0.90–1.32) | 0.373 | 0.97 (0.80–1.19) | 0.787 |
AAD – antiarrhythmic drug; CI – confidence interval; CVH – cardiovascular hospitalization; HR – hazard ratio.
Adjusted results are from Cox proportional hazards regression models.
Multivariable models were adjusted for: age, sex, education status (high school completion), insurance coverage (partial or complete health insurance coverage), body mass index, smoking and drinking status (current smoking and current drinking), history of established coronary artery disease, diabetes mellitus, hypertension, hyperlipidemia, chronic heart failure, previous bleeding, stroke/transient ischemic attack/thromboembolism, abnormal liver function, estimated glomerular filtration rate <60 mL/min/1.73 m2, atrial fibrillation type (persistent atrial fibrillation) and time since atrial fibrillation was diagnosed (≥12 months), hospital level (tertiary hospital) as well as oral anticoagulant use and treatment site (inpatients) at the penultimate follow-up;
Incidence rate presents the number of events per 100 person-years follow-up;
Hazard ratio (HR) for AAD relative to non-AAD usage.
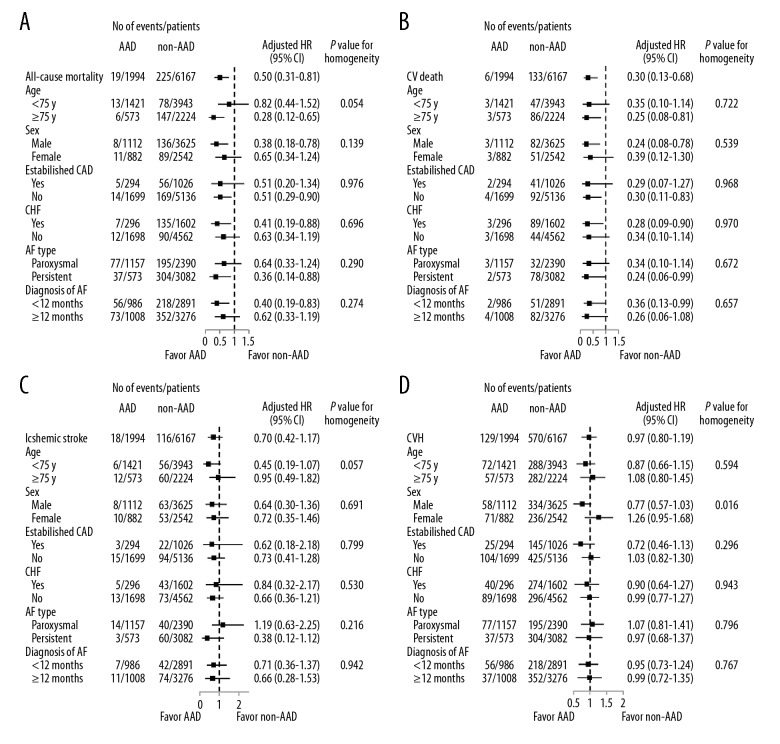
Sensitivity analyses with prolonged observation period showed borderline significant association between AAD usage and overall mortality and cardiovascular death, although AAD was associated with more CVH (Supplementary Table 2). We found higher risk of CVH among female users of AAD, however, there was no evidence of heterogeneity in the effect of AAD use for other outcomes (Figure 3A–3D).
Figure 3.
Subgroup analysis for clinical outcomes in NVAF. Forest plots for all-cause mortality (A), cardiovascular death (B), ischemic stroke (C) and CVH (D) within subgroups defined by age, sex, prior CAD and CHF among patients with NVAF enrolled in China-AF registry between 2008 and 2017 by AAD use. Models were adjusted for: age, sex, education status (high school completion), insurance coverage (partial or complete health insurance coverage), body mass index, smoking and drinking status (current smoking and current drinking), history of established CAD, diabetes mellitus, hypertension, hyperlipidemia, CHF, previous bleeding, stroke/transient ischemic attack/thromboembolism, abnormal liver function, estimated glomerular filtration rate <60 mL/min/1.73 m2, AF type (persistent AF) and time since AF was diagnosed (≥12 months), hospital level (tertiary hospital) as well as oral anticoagulant use and treatment site (inpatients) at the penultimate follow-up. NVAF – non-valvular atrial fibrillation; CVH – cardiovascular hospitalization; CAD – coronary artery disease; CHF – chronic heart failure; China-AF – China Atrial Fibrillation; AAD – antiarrhythmic drugs.
The prevalence of sinus rhythm was 41.6% at the penultimate follow-up, higher in the AAD group than the non-AAD group (46.3% versus 40.1%, P<0.001, Supplementary Table 3).
Discussion
Results of the pivotal AFFIRM trial [5] led to guideline changes and a shift towards rate control strategy in AF patients during the past couple of decades [26]. Our present investigation using contemporary real-word data from the China-AF registry showed an association between AAD usage and lower 1-year overall mortality and cardiovascular death in NVAF patients using an “as treated” exposure definition. We observed no relationship between AAD usage and risk of ischemic stroke or CVH at 1 year. Sensitivity analyses with longer follow-up period showed a trend towards lower overall mortality associated with AAD usage.
The present finding of an association between AAD usage and lower overall mortality in AF patients contrasts with the results of the landmark AFFIRM trial, which may be attributed to a few important reasons. Firstly, the effectiveness and safety profile of AADs vary with the type and extent of concomitant cardiovascular diseases, and younger and healthier patients might benefit preferentially from rhythm control [13,14]. The China-AF study patients on AAD therapy were younger (66.9 years old versus 69.7 years old) and had lower proportion of patients with established CAD (14.8% versus 27.6%) and CHF (14.8% versus 22.8%) in comparison with the AFFIRM cohort [5]. Moreover, our study also had fewer number of patients with new-onset AF (with worse prognosis than those with paroxysmal and persistent AF [27]) than the AFFIRM trial (13.2% versus 35.3%). Secondly, there were variations in the medication usage between our study and the AFFIRM trial. Sotalol [19] and digoxin [28,29] both with increased risk of mortality, were significantly less often used by patients in the China-AF study than the AFFIRM trial (4.7% versus 41.4% and 7.2% versus 32.9%, respectively). The present finding of survival benefit associated with AAD usage corroborates results of prior observational studies. The Quebec study [17] reported that rhythm control was associated with lower mortality after 5 years of AAD initiation [HR 0.77 (0.62–0.95)] and the ORBIT-AF Registry [15] reported borderline survival benefit for patients with rhythm control [HR 0.87 (0.72–1.04)], who had higher rate of comorbid CAD (33.5%) and heart failure (26.1%).
Relating to the secondary outcomes, an earlier observational study had reported a non-significant trend of lower rate of cardiovascular deaths associated with rhythm control [30], whilst the ORBIT-AF Registry [15] showed no significant association between AAD usage and cardiovascular death. The number of cardiovascular deaths in the present study was relatively low, therefore the association between AAD usage and cardiovascular deaths should be interpreted cautiously. Our finding of a lack of relationship between AAD and ischemic stroke is similar to prior results from clinical trials [5,7,11] and the ORBIT-AF registry study [15]. Our study showed that AAD usage was not associated with CVH in the 1-year follow-up period, however, the association became significant when the follow-up period of non-AAD was prolonged to 2 years or more in the sensitivity analyses, similar to results of a previous trial [31] and registries [15,30].
Strengths and limitations
We restricted our sample to AF without reversible causes, included patients who were AAD-naive prior to registry enrollment, eliminated underlying immortal time bias, adjusted for potential baseline confounders and time-dependent covariates such as OAC use and patient treatment site during follow-up. However, there might still be residual confounding. Also, as our study was observational in nature, a causal relationship between AAD usage and lower risk of overall mortality or AAD usage and cardiovascular death should not be inferred. Effects of individual AADs on the clinical outcome of NVAF patients were not evaluated because of the relatively small sample size. Compared to the western populations, the rates of OACs usage for stroke prevention had been lower among the Chinese NVAF patients [22,32,33]. Fortunately, an improvement was observed in recent years in China-AF study [22]. As cardioversion was rarely used, we did not adjust for its effect when we evaluated the association between AAD usage and the prognostic outcome of NVAF patients. Moreover, we did not account for the severity of AF symptoms in our analyses which could also affect patient outcomes. AF can be regarded as a continuous quantitative entity by considering AF burden [34,35] rather than a binary condition (presence or absence of AF) and higher AF burden is associated with higher risk of stroke and mortality, however, this was not investigated in the current study. Finally, our study was conducted primarily in Chinese patients who resided in Beijing; therefore, the results may not be generalizable to other populations.
Conclusions
Our study showed that AAD usage was associated with lower risk of 1-year all-cause mortality and cardiovascular death compared with non-AAD strategy in AF patients. An up-to-date randomized trial to compare rhythm control and rate control strategy in AF patients is warranted.
Supplementary Tables
Supplementary Table 1.
Univariate and multivariate regression of effects of AADs on 1-year outcomes of patients with NVAF.
| Characteristics* | N | All-cause mortality (N=244) | Cardiovascular death (N=139) | ||||
|---|---|---|---|---|---|---|---|
| n/N (%) | Unadjusted HR (95% CI) P value |
Adjusted HR& (95% CI) P value |
n/N (%) | Unadjusted HR (95% CI) P value |
Adjusted HR& (95% CI) P value |
||
| Age, mean (SD), years | 7702 | 75.8±10.4 (244) | 1.08 (1.06–1.09) <0.001 |
1.04 (1.02–1.06) <0.001 |
76.1±9.7 (139) | 1.08 (1.06–1.1) <0.001 |
1.04 (1.02–1.06) <0.001 |
| Men | 8161 | 144/4737 (3.04) | 1.04 (0.81–1.34) 0.770 |
1.25 (0.94–1.67) 0.125 |
85/4737 (1.79) | 1.14 (0.81–1.6) 0.465 |
1.55 (1.06–2.26) 0.022 |
| Completed high school | 7335 | 38/2054 (1.85) | 0.58 (0.41–0.82) 0.002 |
0.77 (0.53–1.12) 0.172 |
19/2054 (0.93) | 0.48 (0.29–0.78) 0.003 |
0.70 (0.4–1.2) 0.193 |
| Partially or complete health insurance coverage | 8156 | 226/7524 (3) | 1.05 (0.65–1.7) 0.837 |
0.89 (0.55–1.46) 0.656 |
126/7524 (1.67) | 0.81 (0.46–1.44) 0.477 |
0.63 (0.35–1.14) 0.128 |
| BMI, mean (SD), kg/m2 | 7080 | 23.7±3.7 (202) | 0.87 (0.84–0.91) <0.001 |
0.92 (0.89–0.96) <0.001 |
23.5±3.6 (115) | 0.86 (0.82–0.91) <0.001 |
0.90 (0.86–0.95) <0.001 |
| Current smoking | 8090 | 39/1253 (3.11) | 1.08 (0.77–1.53) 0.651 |
1.41 (0.94–2.1) 0.096 |
22/1253 (1.76) | 1.08 (0.68–1.7) 0.754 |
1.41 (0.83–2.38) 0.202 |
| Current drinking | 8083 | 31/1493 (2.08) | 0.68 (0.46–0.99) 0.042 |
0.84 (0.54–1.31) 0.445 |
14/1493 (0.94) | 0.52 (0.3–0.91) 0.021 |
0.58 (0.31–1.11) 0.102 |
| Established CAD** | 8155 | 61/1320 (4.62) | 1.70 (1.27–2.27) <0.001 |
1.19 (0.88–1.61) 0.261 |
43/1320 (3.26) | 2.29 (1.6–3.27) <0.001 |
1.55 (1.07–2.26) 0.022 |
| DM | 8157 | 88/2255 (3.9) | 1.44 (1.11–1.87) 0.006 |
1.12 (0.85–1.47) 0.418 |
54/2255 (2.39) | 1.62 (1.15–2.28) 0.006 |
1.14 (0.8–1.64) 0.463 |
| Hypertension | 8157 | 178/5673 (3.14) | 1.15 (0.87–1.52) 0.336 |
0.75 (0.56–1.02) 0.069 |
106/5673 (1.87) | 1.37 (0.93–2.02) 0.117 |
0.86 (0.57–1.31) 0.486 |
| Hyperlipidemia | 3591 | 87/3591 (2.42) | 0.71 (0.54–0.92) 0.009 |
0.71 (0.54–0.93) 0.014 |
56/3591 (1.56) | 0.86 (0.61–1.21) 0.381 |
0.84 (0.59–1.2) 0.334 |
| CHF | 8158 | 142/1898 (7.48) | 4.38 (3.39–5.64) <0.001 |
1.84 (1.38–2.45) <0.001 |
92/1898 (4.85) | 6.14 (4.32–8.73) <0.001 |
2.33 (1.57–3.46) <0.001 |
| Previous bleeding | 8154 | 20/412 (4.85) | 1.65 (1.05–2.61) 0.031 |
1.05 (0.65–1.69) 0.836 |
12/412 (2.91) | 1.75 (0.97–3.16) 0.064 |
1.02 (0.55–1.88) 0.962 |
| Previous stroke/TIA/TE | 8155 | 85/1676 (5.07) | 2.05 (1.57–2.66) <0.001 |
1.33 (1.01–1.75) 0.044 |
55/1676 (3.28) | 2.50 (1.78–3.52) <0.001 |
1.51 (1.06–2.15) 0.023 |
| Abnormal liver function# | 5780 | 28/260 (10.77) | 3.48 (2.34–5.18) <0.001 |
2.54 (1.69–3.8) <0.001 |
18/260 (6.92) | 3.94 (2.39–6.5) <0.001 |
2.93 (1.73–4.96) <0.001 |
| eGFR <60 mL/min/1.73 m2## | 5731 | 45/408 (11.03) | 3.61 (2.59–5.02) <0.001 |
2.04 (1.41–2.93) <0.001 |
28/408 (6.86) | 3.85 (2.53–5.87) <0.001 |
1.98 (1.25–3.14) 0.004 |
| Persistent AF | 8161 | 131/3655 (3.58) | 1.32 (1.03–1.7) 0.030 |
1.16 (0.88–1.53) 0.288 |
80/3655 (2.19) | 1.55 (1.1–2.16) 0.011 |
1.19 (0.82–1.72) 0.368 |
| Diagnosis of AF ≥12 months | 8161 | 139/4284 (3.24) | 1.18 (0.91–1.52) 0.207 |
1.06 (0.8–1.39) 0.692 |
86/4284 (2.01) | 1.44 (1.02–2.03) 0.036 |
1.24 (0.86–1.8) 0.255 |
| Tertiary hospital | 8161 | 120/6381 (1.88) | 0.28 (0.22–0.36) <0.001 |
0.59 (0.44–0.78) <0.001 |
60/6381 (0.94) | 0.22 (0.16–0.31) <0.001 |
0.48 (0.33–0.69) <0.001 |
| OAC at penultimate follow-up | 8161 | 35/2513 (1.39) | 0.36 (0.25–0.51) <0.001 |
0.48 (0.33–0.71) <0.001 |
23/2513 (0.92) | 0.43 (0.27–0.67) <0.001 |
0.65 (0.4–1.05) 0.077 |
| Inpatients at penultimate follow-up | 8160 | 97/1068 (9.08) | 6.32 (4.89–8.18) <0.001 |
4.52 (3.44–5.93) <0.001 |
55/1068 (5.15) | 6.31 (4.48–8.87) <0.001 |
4.08 (2.85–5.85) <0.001 |
| AAD | 8161 | 19/1994 (0.95) | 0.41 (0.26–0.66) <0.001 |
0.50 (0.31–0.81) 0.005 |
6/1994 (0.3) | 0.22 (0.1–0.5) <0.001 |
0.30 (0.13–0.68) 0.004 |
| Age, mean (SD), years | 8161 | 74.1±9.1 (134) | 1.05 (1.04–1.07) <0.001 |
1.03 (1.01–1.05) 0.005 |
72.1±10.4 (699) | 1.03 (1.03–1.04) <0.001 |
1.01 (1–1.01) 0.098 |
| Men | 7335 | 71/4737 (1.5) | 0.81 (0.58–1.14) 0.229 |
0.91 (0.62–1.32) 0.606 |
392/4737 (8.28) | 0.92 (0.79–1.07) 0.289 |
1.12 (0.95–1.33) 0.169 |
| Completed high school | 8156 | 31/2054 (1.51) | 0.91 (0.6–1.37) 0.642 |
0.99 (0.64–1.53) 0.971 |
118/2054 (5.74) | 0.64 (0.53–0.79) <0.001 |
0.82 (0.65–1.02) 0.079 |
| Partially or complete health insurance coverage | 7080 | 122/7524 (1.62) | 0.85 (0.47–1.54) 0.594 |
0.71 (0.39–1.3) 0.267 |
656/7524 (8.72) | 1.29 (0.95–1.76) 0.104 |
1.05 (0.77–1.44) 0.749 |
| BMI, mean (SD), kg/m2 | 7702 | 24.6±3.7 (115) | 0.94 (0.89–0.99) 0.023 |
0.95 (0.9–1.01) 0.086 |
25.5±3.8 (616) | 1.00 (0.98–1.03) 0.725 |
1.01 (0.99–1.03) 0.346 |
| Current smoking | 8161 | 12/1253 (0.96) | 0.55 (0.3–0.99) 0.045 |
0.72 (0.37–1.4) 0.334 |
88/1253 (7.02) | 0.80 (0.64–1) 0.046 |
0.93 (0.72–1.2) 0.557 |
| Current drinking | 8090 | 18/1493 (1.21) | 0.70 (0.43–1.16) 0.164 |
1.22 (0.68–2.18) 0.499 |
95/1493 (6.36) | 0.71 (0.57–0.88) 0.002 |
0.88 (0.69–1.14) 0.340 |
| Established CAD** | 8083 | 25/1320 (1.89) | 1.18 (0.76–1.81) 0.468 |
0.88 (0.56–1.37) 0.572 |
170/1320 (12.88) | 1.68 (1.41–2) <0.001 |
1.17 (0.98–1.4) 0.082 |
| DM | 8155 | 45/2255 (2) | 1.30 (0.91–1.85) 0.157 |
1.01 (0.69–1.47) 0.962 |
257/2255 (11.4) | 1.51 (1.29–1.76) <0.001 |
1.07 (0.92–1.26) 0.383 |
| Hypertension | 8157 | 114/5673 (2.01) | 2.45 (1.52–3.93) <0.001 |
1.94 (1.18–3.2) 0.009 |
568/5673 (10.01) | 1.90 (1.57–2.29) <0.001 |
1.44 (1.17–1.76) <0.001 |
| Hyperlipidemia | 8157 | 57/3591 (1.59) | 0.94 (0.67–1.32) 0.723 |
0.88 (0.62–1.26) 0.497 |
327/3591 (9.11) | 1.12 (0.97–1.3) 0.126 |
0.98 (0.84–1.14) 0.761 |
| CHF | 8158 | 48/1898 (2.53) | 1.77 (1.24–2.52) 0.002 |
1.16 (0.78–1.72) 0.463 |
314/1898 (16.54) | 2.69 (2.32–3.13) <0.001 |
1.75 (1.48–2.08) <0.001 |
| Previous bleeding | 3591 | 11/412 (2.67) | 1.67 (0.9–3.1) 0.103 |
1.16 (0.62–2.18) 0.635 |
47/412 (11.41) | 1.36 (1.01–1.83) 0.043 |
1.07 (0.79–1.45) 0.644 |
| Previous stroke/TIA/TE | 8155 | 61/1610 (3.79) | 3.40 (2.42–4.78) <0.001 |
2.54 (1.78–3.63) <0.001 |
214/1610 (13.29) | 1.82 (1.55–2.14) <0.001 |
1.29 (1.09–1.52) 0.003 |
| Abnormal liver functio# | 8154 | 7/260 (2.69) | 1.86 (0.86–4.02) 0.115 |
2.03 (0.95–4.33) 0.066 |
38/260 (14.62) | 1.71 (1.23–2.38) 0.001 |
1.59 (1.16–2.17) 0.004 |
| eGFR <60 mL/min/1.73 m2## | 8161 | 7/408 (1.72) | 1.05 (0.49–2.27) 0.900 |
0.52 (0.22–1.25) 0.139 |
79/408 (19.36) | 2.30 (1.81–2.92) <0.001 |
1.45 (1.13–1.85) 0.003 |
| Persistent AF | 8161 | 63/3655 (1.72) | 1.02 (0.73–1.43) 0.908 |
0.85 (0.59–1.23) 0.401 |
341/3655 (9.33) | 1.09 (0.94–1.27) 0.236 |
0.91 (0.77–1.07) 0.248 |
| Diagnosis of AF ≥12 months | 5731 | 85/4284 (1.98) | 1.55 (1.09–2.2) 0.015 |
1.44 (1–2.09) 0.051 |
425/4284 (9.92) | 1.39 (1.2–1.62) <0.001 |
1.35 (1.15–1.58) <0.001 |
| Tertiary hospital | 5780 | 96/6381 (1.5) | 0.72 (0.5–1.05) 0.091 |
1.19 (0.79–1.8) 0.414 |
446/6381 (6.99) | 0.49 (0.42–0.58) <0.001 |
0.76 (0.63–0.9) 0.002 |
| OAC at penultimate follow-up | 8160 | 35/2504 (1.4) | 0.76 (0.52–1.12) 0.167 |
0.70 (0.47–1.06) 0.089 |
192/2469 (7.78) | 0.82 (0.7–0.97) 0.022 |
0.91 (0.77–1.09) 0.317 |
| Inpatients at penultimate follow-up | 8161 | 43/1057 (4.07) | 4.53 (3.14–6.52) <0.001 |
3.78 (2.59–5.53) <0.001 |
243/940 (25.85) | 8.27 (7.06–9.7) <0.001 |
6.88 (5.82–8.13) <0.001 |
| AAD | 8161 | 18/1994 (0.9) | 0.73 (0.44–1.2) 0.207 |
0.70 (0.42–1.17) 0.176 |
129/1994 (6.47) | 1.09 (0.9–1.32) 0.373 |
0.97 (0.8–1.19) 0.787 |
AAD – antiarrhythmic drug; AF – atrial fibrillation; BMI – body mass index; CA – coronary artery disease; CHF – chronic heart failure; CI – confidence interval; CVH – cardiovascular hospitalization; DM – diabetes mellitus; eGFR – estimated glomerular filtration rate; HR – hazard ratio; NVAF – nonvalvular atrial fibrillation; OAC – oral anticoagulants; SD – standard deviation; TE – thromboembolism; TIA – transient ischemic attack.
Continuous variables were presented as mean (SD) and categorical variables were presented as number (percent);
Established CAD includes myocardial infarction, percutaneous coronary intervention, and coronary artery bypass grafting;
Liver function was obtained in 5780 patients (1362 in the AAD group and 4418 in the non-AAD group).
Abnormal liver function was defined as serum level of aspartate aminotransferase or alanine aminotransferase >120 U/L, and total bilirubin >34.2 μmol/L;
eGFR was obtained in 5731 patients (1342 in the AAD group and 4389 in the non-AAD group). eGFR (mL/min/1.73 m2)=186×(SCr [μmol/L]×0.0113)−1.154×age−0.203×0.742 (if female);
Multivariable models were adjusted for: age, sex, education status (high school completion), insurance coverage (partial or complete health insurance coverage), body mass index, smoking and drinking status (current smoking and current drinking), history of established coronary artery disease, diabetes mellitus, hypertension, hyperlipidemia, chronic heart failure, previous bleeding, stroke/transient ischemic attack/thromboembolism, abnormal liver function, estimated glomerular filtration rate <60 mL/min/1.73 m2, atrial fibrillation type (persistent atrial fibrillation) and time since atrial fibrillation was diagnosed (≥12 months), hospital level (tertiary hospital) as well as oral anticoagulant use and treatment site (inpatients) at the penultimate follow-up.
Supplementary Table 2.
Association between AAD usage and patient outcomes at 2 years.
| AAD (N=1994) | Non-AAD (N=6167) | Unadjusted results | Adjusted results* | |||||
|---|---|---|---|---|---|---|---|---|
| Events | Rate** | Events | Rate | HR# (95% CI) | P value | HR (95% CI) | P value | |
| All-cause mortality | 37 | 2.09 | 422 | 4.14 | 0.56 (0.40–0.79) | 0.001 | 0.75 (0.53–1.06) | 0.103 |
| Cardiovascular death | 17 | 0.96 | 229 | 2.25 | 0.47 (0.29–0.77) | 0.000 | 0.68 (0.45–1.03) | 0.093 |
| Ischemic stroke | 26 | 1.47 | 183 | 1.82 | 0.83 (0.55–1.25) | 0.366 | 0.84 (0.55–1.29) | 0.424 |
| CVH | 181 | 10.51 | 878 | 9.28 | 1.21 (1.03–1.42) | 0.023 | 1.21 (1.02–1.43) | 0.029 |
AAD – antiarrhythmic drug; CI – confidence interval; CVH – cardiovascular hospitalization; HR – hazard ratio.
Adjusted results are from Cox proportional hazards regression models.
Multivariable models were adjusted for: age, sex, education status (high school completion), insurance coverage (partial or complete health insurance coverage), body mass index, smoking and drinking status (current smoking and current drinking), history of established coronary artery disease, diabetes mellitus, hypertension, hyperlipidemia, chronic heart failure, previous bleeding, stroke/transient ischemic attack/thromboembolism, abnormal liver function, estimated glomerular filtration rate <60 mL/min/1.73 m2, atrial fibrillation type (persistent atrial fibrillation) and time since atrial fibrillation was diagnosed (≥12 months), hospital level (tertiary hospital) as well as oral anticoagulant use and treatment site (inpatients) at the penultimate follow-up;
Incidence rate presents the number of events per 100 subjects-years follow-up;
Hazard ratio (HR) is for AAD relative to non-AAD.
Supplementary Table 3.
Sinus rhythm profile at penultimate follow-up.
| Characteristics | Overall (N=8161) | AAD group (N=1994) | Non-AAD group (N=6167) | P value |
|---|---|---|---|---|
| Sinus rhythm | 3398/8161 (41.6) | 923/1994 (46.3) | 2475/6167 (40.1) | <0.001 |
| Age (years) | ||||
| <65 | 1283/2809 (45.7) | 364/763 (47.7) | 919/2046 (44.9) | 0.187 |
| ≥65 | 2119/5352 (39.6) | 531/1231 (43.1) | 1588/4121 (38.5) | 0.004 |
| Sex | ||||
| Male | 1979/4737 (41.8) | 518/1112 (46.6) | 1461/3625 (40.3) | <0.001 |
| Female | 1423/3424 (41.6) | 377/882 (42.7) | 1046/2542 (41.2) | 0.408 |
| Established CAD | ||||
| Yes | 550/1320 (41.7) | 147/294 (50.0) | 403/1026 (39.3) | 0.001 |
| No | 2849/6835 (41.7) | 748/1699 (44.0) | 2101/5136 (40.9) | 0.024 |
| CHF | ||||
| Yes | 717/1898 (37.8) | 109/296 (36.8) | 608/1602 (38.0) | 0.713 |
| No | 2683/6260 (42.9) | 786/1698 (46.3) | 1897/4562 (41.6) | <0.001 |
| First diagnosis of AF | ||||
| <12 months | 1832/3877 (47.3) | 486/986 (49.3) | 1346/2891 (46.6) | 0.138 |
| ≥12 months | 1570/4284 (36.6) | 409/1008 (40.6) | 1161/3276 (35.4) | 0.003 |
AAD – antiarrhythmic drug; AF – atrial fibrillation; CAD – coronary artery disease; CHF – chronic heart failure.
Acknowledgements
This study was based in part on data from the Chinese Atrial Fibrillation Registry (China-AF). We would like to thank the China-AF investigators for assistance in the data collection. Dr J-Z. Dong received honoraria from Johnson & Johnson for giving lectures. Dr X. Du received honoraria from Bayer for giving lectures.
Footnotes
Source of support: This work was supported by National Key R&D Program of China (2016YFC1301002 and 2016YFC0900901, 2017YFC0908803, 2018YFC1312501), National Natural Science Foundation of China (81530016), and grants (D151100002215003 and D151100002215004) from Beijing Municipal Commission of Science and Technology. The construction of the China-AF was also supported by grants from Bristol-Myers Squibb (BMS), Pfizer, Johnson & Johnson, Boehringer-Ingelheim (BI), and Bayer
Statement
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or production of manuscript.
Trial registration
Chinese Clinical Trial Registry (No. ChiCTR-OCH-13003729); http://www.chictr.org.cn/showproj.aspx?proj=5831
References
- 1.Miyasaka Y, Barnes ME, Petersen RC, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a community-based cohort. Eur Heart J. 2007;28(16):1962–67. doi: 10.1093/eurheartj/ehm012. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 study. Circulation. 2014;129(8):837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. 2014;64(21):e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa S, Yamashita T, Yamazaki T, et al. Optimal treatment strategy for patients with paroxysmal atrial fibrillation: J-RHYTHM Study. Circ J. 2009;73(2):242–48. doi: 10.1253/circj.cj-08-0608. [DOI] [PubMed] [Google Scholar]
- 7.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347(23):1834–40. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 8.Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: The Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41(10):1690–96. doi: 10.1016/s0735-1097(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 9.Opolski G, Torbicki A, Kosior DA, et al. Rate control vs. rhythm control in patients with nonvalvular persistent atrial fibrillation: The results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) study. Chest. 2004;126(2):476–86. doi: 10.1378/chest.126.2.476. [DOI] [PubMed] [Google Scholar]
- 10.Yildiz A, Yigit Z, Okcun B, et al. Comparison of rate and rhythm control in hypertension patients with atrial fibrillation. Circ J. 2008;72(5):705–8. doi: 10.1253/circj.72.705. [DOI] [PubMed] [Google Scholar]
- 11.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 12.Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med. 2016;374(20):1911–21. doi: 10.1056/NEJMoa1602002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee S, Sardar P, Lichstein E, et al. Pharmacologic rate versus rhythm-control strategies in atrial fibrillation: An updated comprehensive review and meta-analysis. Pacing Clin Electrophysiol. 2013;36(1):122–33. doi: 10.1111/j.1540-8159.2012.03513.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Dong Y, Fan J, Yin Y. Rate vs. rhythm control in patients with atrial fibrillation – an updated meta-analysis of 10 randomized controlled trials. Int J Cardiol. 2011;153(1):96–98. doi: 10.1016/j.ijcard.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Noheria A, Shrader P, Piccini JP, et al. Rhythm control versus rate control and clinical outcomes in patients with atrial fibrillation. JACC: Clinical Electrophysiology. 2016;2(2):221–29. doi: 10.1016/j.jacep.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Purmah Y, Proietti M, Laroche C, et al. Rate vs. rhythm control and adverse outcomes among European patients with atrial fibrillation. Europace. 2018;20(2):243–52. doi: 10.1093/europace/euw421. [DOI] [PubMed] [Google Scholar]
- 17.Ionescu-Ittu R, Abrahamowicz M, Jackevicius CA, et al. Comparative effectiveness of rhythm control vs. rate control drug treatment effect on mortality in patients with atrial fibrillation. Arch Intern Med. 2012;172(13):997–1004. doi: 10.1001/archinternmed.2012.2266. [DOI] [PubMed] [Google Scholar]
- 18.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12(10):1360–420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 19.Dan GA, Martinez-Rubio A, Agewall S, et al. Antiarrhythmic drugs-clinical use and clinical decision making: A consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP) Europace. 2018;20(5):731–32. doi: 10.1093/europace/eux373. [DOI] [PubMed] [Google Scholar]
- 20.Lafuente-Lafuente C, Valembois L, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2015;(3):CD005049. doi: 10.1002/14651858.CD005049.pub4. [DOI] [PubMed] [Google Scholar]
- 21.Du X, Ma C, Wu J, et al. Rationale and design of the Chinese Atrial Fibrillation Registry Study. BMC Cardiovasc Disord. 2016;16:130. doi: 10.1186/s12872-016-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang SS, Dong JZ, Ma CS, et al. Current status and time trends of oral anticoagulation use among Chinese patients with nonvalvular atrial fibrillation: The Chinese Atrial Fibrillation Registry Study. Stroke. 2016;47(7):1803–10. doi: 10.1161/STROKEAHA.116.012988. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA Key Data elements and definitions for cardiovascular endpoint events in clinical trials: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards) J Am Coll Cardiol. 2015;66(4):403–69. doi: 10.1016/j.jacc.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492–99. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 26.Snow V, Weiss KB, LeFevre M, et al. Management of newly detected atrial fibrillation: A clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Intern Med. 2003;139(12):1009–17. doi: 10.7326/0003-4819-139-12-200312160-00011. [DOI] [PubMed] [Google Scholar]
- 27.Nieuwlaat R, Prins MH, Le Heuzey JY, et al. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: Follow-up of the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2008;29(9):1181–89. doi: 10.1093/eurheartj/ehn139. [DOI] [PubMed] [Google Scholar]
- 28.Chao TF, Liu CJ, Tuan TC, et al. Rate-control treatment and mortality in atrial fibrillation. Circulation. 2015;132(17):1604–12. doi: 10.1161/CIRCULATIONAHA.114.013709. [DOI] [PubMed] [Google Scholar]
- 29.Washam JB, Stevens SR, Lokhnygina Y, et al. Digoxin use in patients with atrial fibrillation and adverse cardiovascular outcomes: A retrospective analysis of the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) Lancet. 2015;385(9985):2363–70. doi: 10.1016/S0140-6736(14)61836-5. [DOI] [PubMed] [Google Scholar]
- 30.Camm AJ, Breithardt G, Crijns H, et al. Real-life observations of clinical outcomes with rhythm- and rate-control therapies for atrial fibrillation. J Am Coll Cardiol. 2011;58(5):493–501. doi: 10.1016/j.jacc.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 31.Saksena S, Slee A, Waldo AL, et al. Cardiovascular outcomes in the AFFIRM Trial (Atrial Fibrillation Follow-Up Investigation of Rhythm Management) J Am Coll Cardiol. 2011;58(19):1975–85. doi: 10.1016/j.jacc.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oldgren J, Healey JS, Ezekowitz M, et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15 400 emergency department patients in 46 countries: The RE-LY Atrial Fibrillation Registry. Circulation. 2014;129(15):1568–76. doi: 10.1161/CIRCULATIONAHA.113.005451. [DOI] [PubMed] [Google Scholar]
- 33.Hu D, Sun Y. Epidemiology, risk factors for stroke, and management of atrial fibrillation in China. J Am Coll Cardiol. 2008;52(10):865–68. doi: 10.1016/j.jacc.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 34.Go AS, Reynolds K, Yang J, et al. Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: The KP-RHYTHM Study. JAMA Cardiol. 2018;3(7):601–8. doi: 10.1001/jamacardio.2018.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen LY, Chung MK, Allen LA, et al. Atrial fibrillation burden: Moving beyond atrial fibrillation as a binary entity: A scientific statement from the American Heart Association. Circulation. 2018;137(20):e623–44. doi: 10.1161/CIR.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Univariate and multivariate regression of effects of AADs on 1-year outcomes of patients with NVAF.
| Characteristics* | N | All-cause mortality (N=244) | Cardiovascular death (N=139) | ||||
|---|---|---|---|---|---|---|---|
| n/N (%) | Unadjusted HR (95% CI) P value |
Adjusted HR& (95% CI) P value |
n/N (%) | Unadjusted HR (95% CI) P value |
Adjusted HR& (95% CI) P value |
||
| Age, mean (SD), years | 7702 | 75.8±10.4 (244) | 1.08 (1.06–1.09) <0.001 |
1.04 (1.02–1.06) <0.001 |
76.1±9.7 (139) | 1.08 (1.06–1.1) <0.001 |
1.04 (1.02–1.06) <0.001 |
| Men | 8161 | 144/4737 (3.04) | 1.04 (0.81–1.34) 0.770 |
1.25 (0.94–1.67) 0.125 |
85/4737 (1.79) | 1.14 (0.81–1.6) 0.465 |
1.55 (1.06–2.26) 0.022 |
| Completed high school | 7335 | 38/2054 (1.85) | 0.58 (0.41–0.82) 0.002 |
0.77 (0.53–1.12) 0.172 |
19/2054 (0.93) | 0.48 (0.29–0.78) 0.003 |
0.70 (0.4–1.2) 0.193 |
| Partially or complete health insurance coverage | 8156 | 226/7524 (3) | 1.05 (0.65–1.7) 0.837 |
0.89 (0.55–1.46) 0.656 |
126/7524 (1.67) | 0.81 (0.46–1.44) 0.477 |
0.63 (0.35–1.14) 0.128 |
| BMI, mean (SD), kg/m2 | 7080 | 23.7±3.7 (202) | 0.87 (0.84–0.91) <0.001 |
0.92 (0.89–0.96) <0.001 |
23.5±3.6 (115) | 0.86 (0.82–0.91) <0.001 |
0.90 (0.86–0.95) <0.001 |
| Current smoking | 8090 | 39/1253 (3.11) | 1.08 (0.77–1.53) 0.651 |
1.41 (0.94–2.1) 0.096 |
22/1253 (1.76) | 1.08 (0.68–1.7) 0.754 |
1.41 (0.83–2.38) 0.202 |
| Current drinking | 8083 | 31/1493 (2.08) | 0.68 (0.46–0.99) 0.042 |
0.84 (0.54–1.31) 0.445 |
14/1493 (0.94) | 0.52 (0.3–0.91) 0.021 |
0.58 (0.31–1.11) 0.102 |
| Established CAD** | 8155 | 61/1320 (4.62) | 1.70 (1.27–2.27) <0.001 |
1.19 (0.88–1.61) 0.261 |
43/1320 (3.26) | 2.29 (1.6–3.27) <0.001 |
1.55 (1.07–2.26) 0.022 |
| DM | 8157 | 88/2255 (3.9) | 1.44 (1.11–1.87) 0.006 |
1.12 (0.85–1.47) 0.418 |
54/2255 (2.39) | 1.62 (1.15–2.28) 0.006 |
1.14 (0.8–1.64) 0.463 |
| Hypertension | 8157 | 178/5673 (3.14) | 1.15 (0.87–1.52) 0.336 |
0.75 (0.56–1.02) 0.069 |
106/5673 (1.87) | 1.37 (0.93–2.02) 0.117 |
0.86 (0.57–1.31) 0.486 |
| Hyperlipidemia | 3591 | 87/3591 (2.42) | 0.71 (0.54–0.92) 0.009 |
0.71 (0.54–0.93) 0.014 |
56/3591 (1.56) | 0.86 (0.61–1.21) 0.381 |
0.84 (0.59–1.2) 0.334 |
| CHF | 8158 | 142/1898 (7.48) | 4.38 (3.39–5.64) <0.001 |
1.84 (1.38–2.45) <0.001 |
92/1898 (4.85) | 6.14 (4.32–8.73) <0.001 |
2.33 (1.57–3.46) <0.001 |
| Previous bleeding | 8154 | 20/412 (4.85) | 1.65 (1.05–2.61) 0.031 |
1.05 (0.65–1.69) 0.836 |
12/412 (2.91) | 1.75 (0.97–3.16) 0.064 |
1.02 (0.55–1.88) 0.962 |
| Previous stroke/TIA/TE | 8155 | 85/1676 (5.07) | 2.05 (1.57–2.66) <0.001 |
1.33 (1.01–1.75) 0.044 |
55/1676 (3.28) | 2.50 (1.78–3.52) <0.001 |
1.51 (1.06–2.15) 0.023 |
| Abnormal liver function# | 5780 | 28/260 (10.77) | 3.48 (2.34–5.18) <0.001 |
2.54 (1.69–3.8) <0.001 |
18/260 (6.92) | 3.94 (2.39–6.5) <0.001 |
2.93 (1.73–4.96) <0.001 |
| eGFR <60 mL/min/1.73 m2## | 5731 | 45/408 (11.03) | 3.61 (2.59–5.02) <0.001 |
2.04 (1.41–2.93) <0.001 |
28/408 (6.86) | 3.85 (2.53–5.87) <0.001 |
1.98 (1.25–3.14) 0.004 |
| Persistent AF | 8161 | 131/3655 (3.58) | 1.32 (1.03–1.7) 0.030 |
1.16 (0.88–1.53) 0.288 |
80/3655 (2.19) | 1.55 (1.1–2.16) 0.011 |
1.19 (0.82–1.72) 0.368 |
| Diagnosis of AF ≥12 months | 8161 | 139/4284 (3.24) | 1.18 (0.91–1.52) 0.207 |
1.06 (0.8–1.39) 0.692 |
86/4284 (2.01) | 1.44 (1.02–2.03) 0.036 |
1.24 (0.86–1.8) 0.255 |
| Tertiary hospital | 8161 | 120/6381 (1.88) | 0.28 (0.22–0.36) <0.001 |
0.59 (0.44–0.78) <0.001 |
60/6381 (0.94) | 0.22 (0.16–0.31) <0.001 |
0.48 (0.33–0.69) <0.001 |
| OAC at penultimate follow-up | 8161 | 35/2513 (1.39) | 0.36 (0.25–0.51) <0.001 |
0.48 (0.33–0.71) <0.001 |
23/2513 (0.92) | 0.43 (0.27–0.67) <0.001 |
0.65 (0.4–1.05) 0.077 |
| Inpatients at penultimate follow-up | 8160 | 97/1068 (9.08) | 6.32 (4.89–8.18) <0.001 |
4.52 (3.44–5.93) <0.001 |
55/1068 (5.15) | 6.31 (4.48–8.87) <0.001 |
4.08 (2.85–5.85) <0.001 |
| AAD | 8161 | 19/1994 (0.95) | 0.41 (0.26–0.66) <0.001 |
0.50 (0.31–0.81) 0.005 |
6/1994 (0.3) | 0.22 (0.1–0.5) <0.001 |
0.30 (0.13–0.68) 0.004 |
| Age, mean (SD), years | 8161 | 74.1±9.1 (134) | 1.05 (1.04–1.07) <0.001 |
1.03 (1.01–1.05) 0.005 |
72.1±10.4 (699) | 1.03 (1.03–1.04) <0.001 |
1.01 (1–1.01) 0.098 |
| Men | 7335 | 71/4737 (1.5) | 0.81 (0.58–1.14) 0.229 |
0.91 (0.62–1.32) 0.606 |
392/4737 (8.28) | 0.92 (0.79–1.07) 0.289 |
1.12 (0.95–1.33) 0.169 |
| Completed high school | 8156 | 31/2054 (1.51) | 0.91 (0.6–1.37) 0.642 |
0.99 (0.64–1.53) 0.971 |
118/2054 (5.74) | 0.64 (0.53–0.79) <0.001 |
0.82 (0.65–1.02) 0.079 |
| Partially or complete health insurance coverage | 7080 | 122/7524 (1.62) | 0.85 (0.47–1.54) 0.594 |
0.71 (0.39–1.3) 0.267 |
656/7524 (8.72) | 1.29 (0.95–1.76) 0.104 |
1.05 (0.77–1.44) 0.749 |
| BMI, mean (SD), kg/m2 | 7702 | 24.6±3.7 (115) | 0.94 (0.89–0.99) 0.023 |
0.95 (0.9–1.01) 0.086 |
25.5±3.8 (616) | 1.00 (0.98–1.03) 0.725 |
1.01 (0.99–1.03) 0.346 |
| Current smoking | 8161 | 12/1253 (0.96) | 0.55 (0.3–0.99) 0.045 |
0.72 (0.37–1.4) 0.334 |
88/1253 (7.02) | 0.80 (0.64–1) 0.046 |
0.93 (0.72–1.2) 0.557 |
| Current drinking | 8090 | 18/1493 (1.21) | 0.70 (0.43–1.16) 0.164 |
1.22 (0.68–2.18) 0.499 |
95/1493 (6.36) | 0.71 (0.57–0.88) 0.002 |
0.88 (0.69–1.14) 0.340 |
| Established CAD** | 8083 | 25/1320 (1.89) | 1.18 (0.76–1.81) 0.468 |
0.88 (0.56–1.37) 0.572 |
170/1320 (12.88) | 1.68 (1.41–2) <0.001 |
1.17 (0.98–1.4) 0.082 |
| DM | 8155 | 45/2255 (2) | 1.30 (0.91–1.85) 0.157 |
1.01 (0.69–1.47) 0.962 |
257/2255 (11.4) | 1.51 (1.29–1.76) <0.001 |
1.07 (0.92–1.26) 0.383 |
| Hypertension | 8157 | 114/5673 (2.01) | 2.45 (1.52–3.93) <0.001 |
1.94 (1.18–3.2) 0.009 |
568/5673 (10.01) | 1.90 (1.57–2.29) <0.001 |
1.44 (1.17–1.76) <0.001 |
| Hyperlipidemia | 8157 | 57/3591 (1.59) | 0.94 (0.67–1.32) 0.723 |
0.88 (0.62–1.26) 0.497 |
327/3591 (9.11) | 1.12 (0.97–1.3) 0.126 |
0.98 (0.84–1.14) 0.761 |
| CHF | 8158 | 48/1898 (2.53) | 1.77 (1.24–2.52) 0.002 |
1.16 (0.78–1.72) 0.463 |
314/1898 (16.54) | 2.69 (2.32–3.13) <0.001 |
1.75 (1.48–2.08) <0.001 |
| Previous bleeding | 3591 | 11/412 (2.67) | 1.67 (0.9–3.1) 0.103 |
1.16 (0.62–2.18) 0.635 |
47/412 (11.41) | 1.36 (1.01–1.83) 0.043 |
1.07 (0.79–1.45) 0.644 |
| Previous stroke/TIA/TE | 8155 | 61/1610 (3.79) | 3.40 (2.42–4.78) <0.001 |
2.54 (1.78–3.63) <0.001 |
214/1610 (13.29) | 1.82 (1.55–2.14) <0.001 |
1.29 (1.09–1.52) 0.003 |
| Abnormal liver functio# | 8154 | 7/260 (2.69) | 1.86 (0.86–4.02) 0.115 |
2.03 (0.95–4.33) 0.066 |
38/260 (14.62) | 1.71 (1.23–2.38) 0.001 |
1.59 (1.16–2.17) 0.004 |
| eGFR <60 mL/min/1.73 m2## | 8161 | 7/408 (1.72) | 1.05 (0.49–2.27) 0.900 |
0.52 (0.22–1.25) 0.139 |
79/408 (19.36) | 2.30 (1.81–2.92) <0.001 |
1.45 (1.13–1.85) 0.003 |
| Persistent AF | 8161 | 63/3655 (1.72) | 1.02 (0.73–1.43) 0.908 |
0.85 (0.59–1.23) 0.401 |
341/3655 (9.33) | 1.09 (0.94–1.27) 0.236 |
0.91 (0.77–1.07) 0.248 |
| Diagnosis of AF ≥12 months | 5731 | 85/4284 (1.98) | 1.55 (1.09–2.2) 0.015 |
1.44 (1–2.09) 0.051 |
425/4284 (9.92) | 1.39 (1.2–1.62) <0.001 |
1.35 (1.15–1.58) <0.001 |
| Tertiary hospital | 5780 | 96/6381 (1.5) | 0.72 (0.5–1.05) 0.091 |
1.19 (0.79–1.8) 0.414 |
446/6381 (6.99) | 0.49 (0.42–0.58) <0.001 |
0.76 (0.63–0.9) 0.002 |
| OAC at penultimate follow-up | 8160 | 35/2504 (1.4) | 0.76 (0.52–1.12) 0.167 |
0.70 (0.47–1.06) 0.089 |
192/2469 (7.78) | 0.82 (0.7–0.97) 0.022 |
0.91 (0.77–1.09) 0.317 |
| Inpatients at penultimate follow-up | 8161 | 43/1057 (4.07) | 4.53 (3.14–6.52) <0.001 |
3.78 (2.59–5.53) <0.001 |
243/940 (25.85) | 8.27 (7.06–9.7) <0.001 |
6.88 (5.82–8.13) <0.001 |
| AAD | 8161 | 18/1994 (0.9) | 0.73 (0.44–1.2) 0.207 |
0.70 (0.42–1.17) 0.176 |
129/1994 (6.47) | 1.09 (0.9–1.32) 0.373 |
0.97 (0.8–1.19) 0.787 |
AAD – antiarrhythmic drug; AF – atrial fibrillation; BMI – body mass index; CA – coronary artery disease; CHF – chronic heart failure; CI – confidence interval; CVH – cardiovascular hospitalization; DM – diabetes mellitus; eGFR – estimated glomerular filtration rate; HR – hazard ratio; NVAF – nonvalvular atrial fibrillation; OAC – oral anticoagulants; SD – standard deviation; TE – thromboembolism; TIA – transient ischemic attack.
Continuous variables were presented as mean (SD) and categorical variables were presented as number (percent);
Established CAD includes myocardial infarction, percutaneous coronary intervention, and coronary artery bypass grafting;
Liver function was obtained in 5780 patients (1362 in the AAD group and 4418 in the non-AAD group).
Abnormal liver function was defined as serum level of aspartate aminotransferase or alanine aminotransferase >120 U/L, and total bilirubin >34.2 μmol/L;
eGFR was obtained in 5731 patients (1342 in the AAD group and 4389 in the non-AAD group). eGFR (mL/min/1.73 m2)=186×(SCr [μmol/L]×0.0113)−1.154×age−0.203×0.742 (if female);
Multivariable models were adjusted for: age, sex, education status (high school completion), insurance coverage (partial or complete health insurance coverage), body mass index, smoking and drinking status (current smoking and current drinking), history of established coronary artery disease, diabetes mellitus, hypertension, hyperlipidemia, chronic heart failure, previous bleeding, stroke/transient ischemic attack/thromboembolism, abnormal liver function, estimated glomerular filtration rate <60 mL/min/1.73 m2, atrial fibrillation type (persistent atrial fibrillation) and time since atrial fibrillation was diagnosed (≥12 months), hospital level (tertiary hospital) as well as oral anticoagulant use and treatment site (inpatients) at the penultimate follow-up.
Supplementary Table 2.
Association between AAD usage and patient outcomes at 2 years.
| AAD (N=1994) | Non-AAD (N=6167) | Unadjusted results | Adjusted results* | |||||
|---|---|---|---|---|---|---|---|---|
| Events | Rate** | Events | Rate | HR# (95% CI) | P value | HR (95% CI) | P value | |
| All-cause mortality | 37 | 2.09 | 422 | 4.14 | 0.56 (0.40–0.79) | 0.001 | 0.75 (0.53–1.06) | 0.103 |
| Cardiovascular death | 17 | 0.96 | 229 | 2.25 | 0.47 (0.29–0.77) | 0.000 | 0.68 (0.45–1.03) | 0.093 |
| Ischemic stroke | 26 | 1.47 | 183 | 1.82 | 0.83 (0.55–1.25) | 0.366 | 0.84 (0.55–1.29) | 0.424 |
| CVH | 181 | 10.51 | 878 | 9.28 | 1.21 (1.03–1.42) | 0.023 | 1.21 (1.02–1.43) | 0.029 |
AAD – antiarrhythmic drug; CI – confidence interval; CVH – cardiovascular hospitalization; HR – hazard ratio.
Adjusted results are from Cox proportional hazards regression models.
Multivariable models were adjusted for: age, sex, education status (high school completion), insurance coverage (partial or complete health insurance coverage), body mass index, smoking and drinking status (current smoking and current drinking), history of established coronary artery disease, diabetes mellitus, hypertension, hyperlipidemia, chronic heart failure, previous bleeding, stroke/transient ischemic attack/thromboembolism, abnormal liver function, estimated glomerular filtration rate <60 mL/min/1.73 m2, atrial fibrillation type (persistent atrial fibrillation) and time since atrial fibrillation was diagnosed (≥12 months), hospital level (tertiary hospital) as well as oral anticoagulant use and treatment site (inpatients) at the penultimate follow-up;
Incidence rate presents the number of events per 100 subjects-years follow-up;
Hazard ratio (HR) is for AAD relative to non-AAD.
Supplementary Table 3.
Sinus rhythm profile at penultimate follow-up.
| Characteristics | Overall (N=8161) | AAD group (N=1994) | Non-AAD group (N=6167) | P value |
|---|---|---|---|---|
| Sinus rhythm | 3398/8161 (41.6) | 923/1994 (46.3) | 2475/6167 (40.1) | <0.001 |
| Age (years) | ||||
| <65 | 1283/2809 (45.7) | 364/763 (47.7) | 919/2046 (44.9) | 0.187 |
| ≥65 | 2119/5352 (39.6) | 531/1231 (43.1) | 1588/4121 (38.5) | 0.004 |
| Sex | ||||
| Male | 1979/4737 (41.8) | 518/1112 (46.6) | 1461/3625 (40.3) | <0.001 |
| Female | 1423/3424 (41.6) | 377/882 (42.7) | 1046/2542 (41.2) | 0.408 |
| Established CAD | ||||
| Yes | 550/1320 (41.7) | 147/294 (50.0) | 403/1026 (39.3) | 0.001 |
| No | 2849/6835 (41.7) | 748/1699 (44.0) | 2101/5136 (40.9) | 0.024 |
| CHF | ||||
| Yes | 717/1898 (37.8) | 109/296 (36.8) | 608/1602 (38.0) | 0.713 |
| No | 2683/6260 (42.9) | 786/1698 (46.3) | 1897/4562 (41.6) | <0.001 |
| First diagnosis of AF | ||||
| <12 months | 1832/3877 (47.3) | 486/986 (49.3) | 1346/2891 (46.6) | 0.138 |
| ≥12 months | 1570/4284 (36.6) | 409/1008 (40.6) | 1161/3276 (35.4) | 0.003 |
AAD – antiarrhythmic drug; AF – atrial fibrillation; CAD – coronary artery disease; CHF – chronic heart failure.