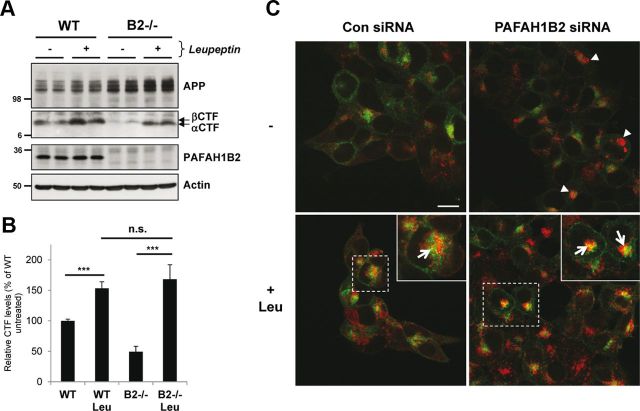
Figure 5.
Loss of PAFAH1B2 increases lysosomal degradation of APP CTFs. A, WT and PAFAH1B2−/− MEFs were treated in the absence or presence of 100 μm of the lysosomal protease inhibitor leupeptin. Treatment resulted in a moderate increase in APP CTFs for WT cells. Untreated knock-out MEFs showed reduced APP CTFs, as before, but during treatment with leupeptin, they were restored to normal levels. B, Quantification of APP CTF immunoblots for nine independent replicates of the experiment performed in A, with APP CTF levels from untreated WT cells set to 100%. The increase in APP CTFs after leupeptin treatment was highly significant for both WT and PAFAH1B2−/− MEFs (***p < 0.001, unpaired Student's t test), whereas the difference in the extent of APP CTF recovery was not significant between the two cell types. C, Confocal microscopy of HEK293–APP cells treated with a nontargeting siRNA pool or an siRNA pool against PAFAH1B2, in the presence or absence of 100 μm leupeptin (Leu). Cells were stained with 6687 polyclonal antibody to the APP C terminus (green) and LAMP2 antibody (red). Knockdown of PAFAH1B2 revealed an increased LAMP2 staining compared with control cells (arrowheads), with some larger clusters clearly visible. Treatment of control and PAFAH1B2 knockdown cells with leupeptin increased LAMP2 staining in both cases, but this increase was more pronounced in PAFAH1B2 knockdown cells. After leupeptin treatment, a partial colocalization of APP with LAMP2 was also observed in both cases, as indicated by the yellow staining (arrows) in the enlarged panels. This partial colocalization was more pronounced after PAFAH1B2 knockdown and leupeptin treatment. Scale bar, 15 μm. Con, Control.