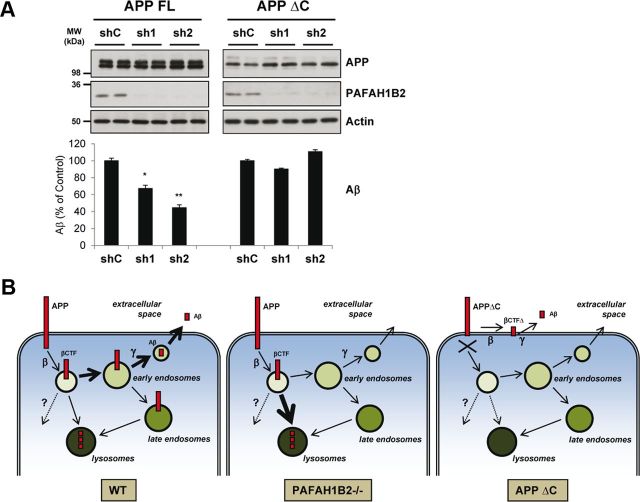
Figure 6.
The APP intracellular domain is required for the PAFAH1B2-mediated effect on Aβ. A, HEK293 cells stably expressing full-length APP (FL) or C-terminally truncated APP (ΔC) were treated with lentiviruses carrying a nontargeting shRNA or shRNAs against PAFAH1B2. Potent knockdowns were achieved in both cell lines, but although this corresponded to reductions in Aβ for cells expressing full-length APP, there was no change in Aβ for cells expressing the truncated version. Protein levels of APP and actin were unaffected in each case. B, Model depicting how PAFAH1B2 can influence Aβ generation. Under WT conditions, APP is internalized from the plasma membrane into vesicles after which it is cleaved by β-secretase to generate the βCTF. Thereafter, a large portion of the βCTF is trafficked through the early endosomes, in which it meets γ-secretase and is cleaved to generate the Aβ peptide, which is then secreted. Another portion of the βCTF is trafficked directly to the lysosomes in which it cannot give rise to Aβ and is degraded. Some CTFs may be degraded by other, as yet undefined, mechanisms as depicted by the dotted line and question mark. Upon loss of PAFAH1B2, the trafficking of βCTFs to the lysosome may be promoted, which results in their enhanced degradation before they can meet γ-secretase for the generation of Aβ. In the case of APPΔC, internalization of the protein is blocked and so any changes in post-endocytic traffic induced by loss of PAFAH1B2 do not affect the generation of Aβ. β-Secretase and γ-secretase can cleave APPΔC to a lesser extent at the plasma membrane as depicted here, or en route to the plasma membrane, to generate Aβ. MW, Molecular weight. Bar graphs show mean and SEM of four individual experiments (*p < 0.05, **p < 0.01, unpaired Student's t test).