Figure 1.
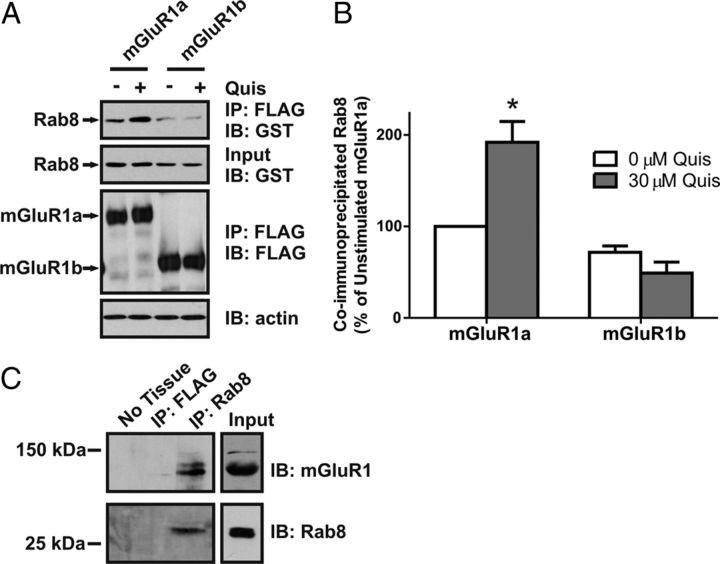
Agonist-dependent coimmunoprecipitation of Rab8 with mGluR1a. A, B, Representative immunoblot (A) and densitometric analysis (B) showing the coimmunoprecipitation of GST-Rab8 with the FLAG-mGluR1a and FLAG-mGluR1b in the absence (−) and presence (+) of 30 μm quisqualate (Quis) treatment for 15 min. HEK 293 cells were transiently transfected with 1 μg of plasmid cDNA encoding either FLAG-mGluR1a or FLAG-mGluR1b along with 2 μg of plasmid cDNA encoding GST-Rab8. Forty-eight hours after transfection, cells were stimulated and lysates were collected and FLAG-mGluR1 was immunoprecipitated. Proteins were separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted for FLAG-mGluR1 and GST-Rab8. Data represent the mean ± SD of three independent experiments. Data were normalized for both GST-Rab protein expression levels and FLAG-mGluR immunoprecipitation and normalized to GST-Rab protein binding to the mGluR1a in absence of agonist. *p < 0.05 compared with GST-Rab8 coimmunoprecipitated with the mGluR1a in the absence of agonist. C, Representative immunoblot showing the coimmunoprecipitation of mGluR1 with Rab8-specific antibody from adult mouse hippocampal lysates.