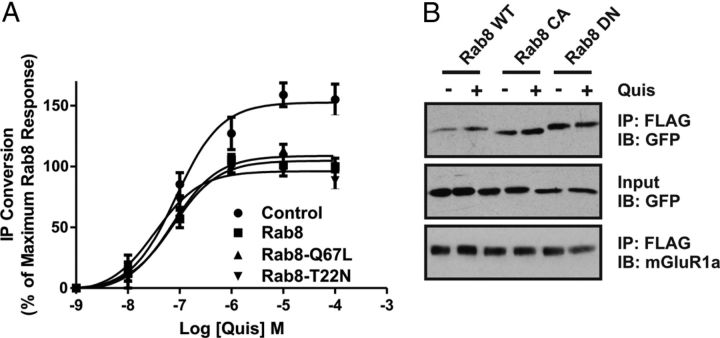
Figure 6.
Effect of Rab8 nucleotide binding mutants on mGluR1a-mediated inositol phosphate formation. A, IP formation in HEK 293 cells transfected with 1 μg of plasmid cDNA encoding FLAG-mGluR1a with 2 μg of plasmid cDNA encoding GFP-tagged wild-type (WT) Rab8, constitutively active (CA) Rab8-Q67L, and dominant-negative (DN) Rab8-T22N mutants. Transfected cells were treated for 10 min with 10 mm LiCl and stimulated with 30 μm quisqualate (Quis) for 30 min in the presence of LiCl. Data were normalized for protein expression and basal IP formation. B, Representative immunoblot showing the coimmunoprecipitation of GST-tagged Rab8WT, Rab8CA and Rab8DN with the FLAG-mGluR1a in the absence (−) and presence (+) of 30 μm Quis treatment for 15 min. HEK 293 cells were transiently transfected with 1 μg of plasmid cDNA encoding FLAG-mGluR1a along with 2 μg of plasmid cDNA encoding GST-tagged Rab8WT, Rab8CA, or Rab8DN. Forty-eight hours after transfection, cells were stimulated, and lysates were collected, separated by SDS-PAGE, transferred to nitrocellulose, and immunoblotted for mGluR1a and GST-Rab8. Data represent the mean ± SD of three to five independent experiments.