Figure 3.
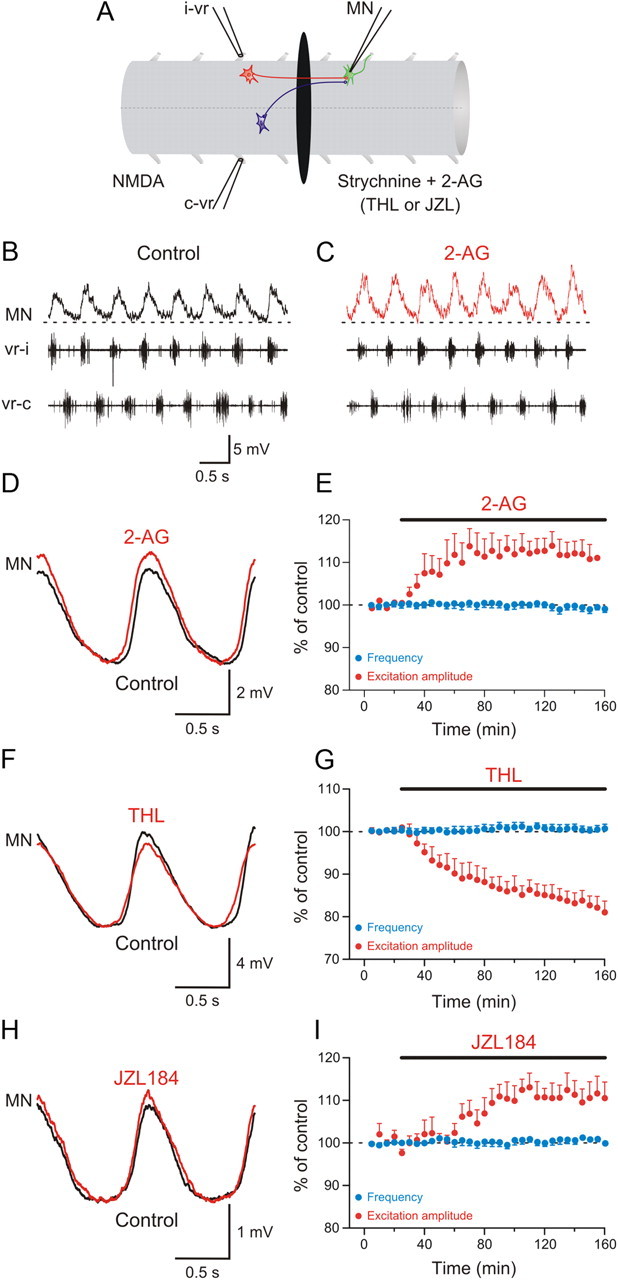
Potentiation of excitatory synaptic transmission by the endocannabinoid 2-AG. A, The recording chamber was divided into two pools with an agar barrier, and locomotor activity was induced by NMDA in the rostral part while locomotor-driven excitation was recorded in a MN in the caudal pool, which was perfused with normal saline solution containing strychnine to block inhibition. B, The recorded MN received excitatory inputs occurring in phase with the ipsilateral ventral root burst. C, Application of 2-AG to the caudal pool increased the amplitude of excitatory synaptic input received by the MN. D, Averaged excitatory synaptic input received by the MN showing an increase in amplitude induced by 2-AG. E, Plot showing average data from different experiments showing the time course of the change in the amplitude of excitation in the caudal pool (red) and the locomotor burst frequency in the rostral pool (blue) before and during 2-AG application. F, Average excitatory synaptic input showing a decrease in amplitude induced by THL. G, Plot of averaged data from different experiments showing the time course of the change in the amplitude of the excitation in the caudal pool (red) and the locomotor burst frequency in the rostral pool (blue) in control and in THL. H, Application of JZL184 in the caudal pool increased the amplitude of the excitatory input received by the MN. I, Plot of averaged data from different experiments showing the change in the amplitude of excitation in the caudal pool (red) and the locomotor burst frequency in the rostral pool (blue) in control and in JZL184.
