Figure 1. Sactipeptide biosynthesis and ring topologies.
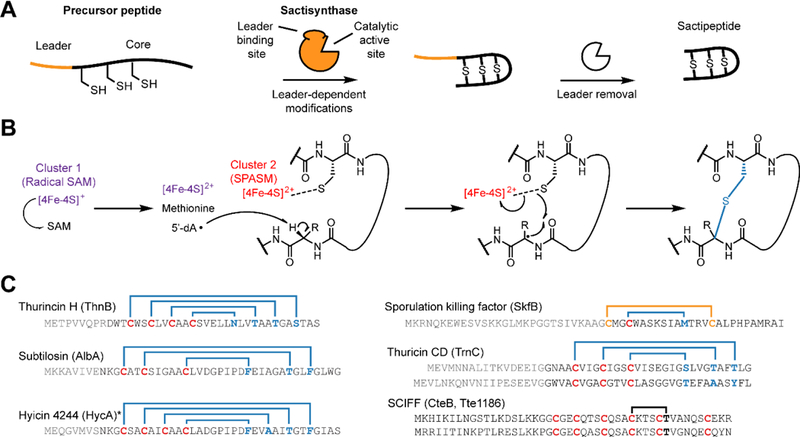
(A) Schematic overview of sactipeptide biosynthesis. (B) Proposed mechanism for the rSAM-dependent formation of the sactipeptide S–Cα thioether crosslink. (C) Precursor peptide sequence and ring topologies of characterized sactipeptides (blue, S–Cα linkage; yellow, S-S linkage; black, uncharacterized). The requisite rSAM enzyme name is given in parentheses. Characterization of the six Cys in forty-five residues (SCIFFs) is limited to in vitro enzymatic assay for which only one thioether linkage was observed. Data shown later in this manuscript support a Cys-Thr linkage different to what has been previously reported and further that SCIFFs are not sactipeptides. * Hyicin 4244 has not been structurally characterized, thus the linkages indicated are speculative.
