Figure 8.
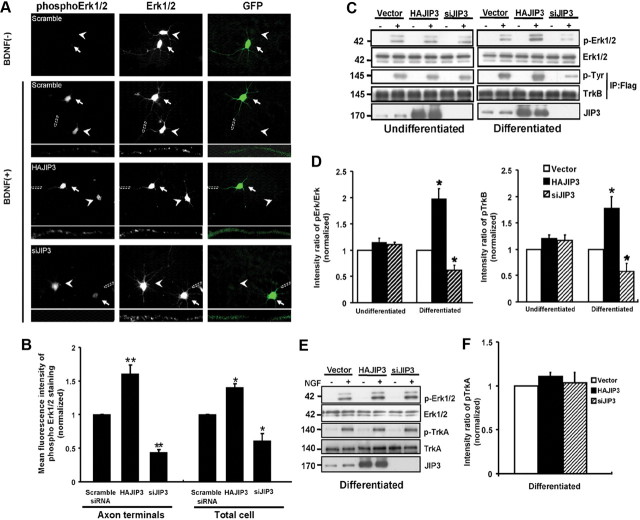
JIP3 is involved in BDNF-induced Erk1/2 phosphorylation in neurons. A, BDNF-induced Erk1/2 phosphorylation in siJIP3- or HAJIP3-transfected neurons. Immunostaining of Erk1/2 and pErk1/2 was performed in cultured neurons. The hippocampal neurons were treated for 15 min with BDNF (50 ng/ml), and levels of total Erk1/2 and pErk1/2 were examined using anti-Erk1/2 and anti-pErk1/2 antibodies. GFP indicates the transfected neurons. Arrowheads indicate the untransfected neurons, and arrows indicate the transfected neurons. Scale bar, 50 μm. Bottom panels show enlarged images of the framed regions indicating immunostaining in axon terminals. B, Quantitative analysis of the mean fluorescence intensity of phosphorylation of Erk1/2 in HAJIP3- or siJIP3-transfected neurons compared with the control condition (scramble siRNA group). Data shown are the mean ± SEM from three independent experiments (n = 3; *p < 0.05; **p < 0.01, vs the scramble siRNA group; one-way ANOVA). C, Immunoblotting using anti-Erk1/2 and anti-pErk1/2 antibodies to assess activation of Erk1/2 in PC12 cells stably expressed TrkB (differentiated or undifferentiated) with JIP3 overexpression or knockdown when treated with BDNF. PhosphoTrkB was also analyzed by immunoprecipitation with rabbit anti-Flag antibodies and immunoblotting with anti-phosphoTyr antibodies. D, Histogram shows pErk1/2 and pTrkB levels in each group in C. Data were shown as the mean ± SEM from three independent experiments (n = 3; *p < 0.05, vs the vector control group; one-way ANOVA). E, Immunoblotting using anti-Erk1/2 and anti-pErk1/2 antibodies to assess activation of Erk1/2 in differentiated PC12 cells (with endogenous expression of TrkA) with JIP3 overexpression or knockdown when treated with NGF (50 ng/ml). PhosphoTrkA was also analyzed by immunoblotting. F, Histogram shows pErk1/2 and pTrkA levels in each group in E. Data were shown as the mean ± SEM from three independent experiments.