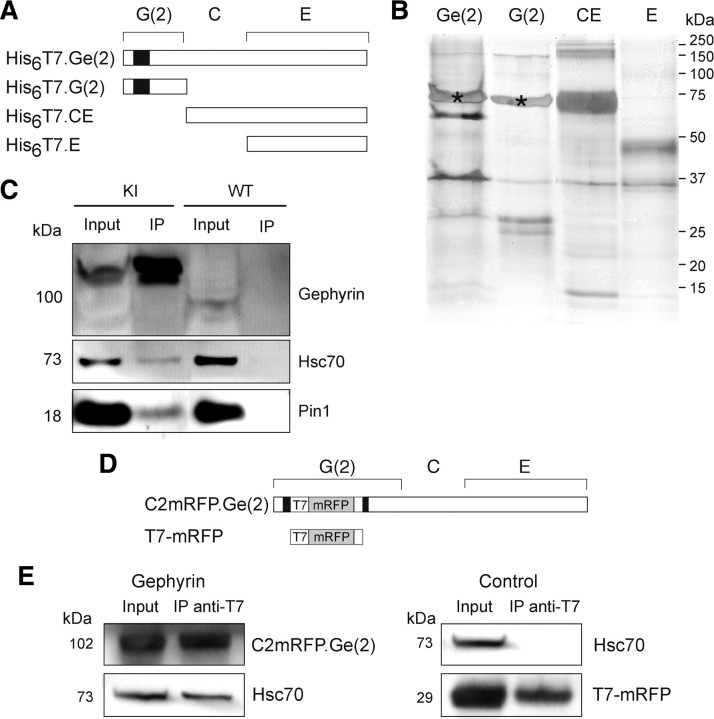
Figure 1.
Gephyrin and Hsc70 interact in vitro and form complexes in COS-7 cells. A, Rod structure of tagged-recombinant gephyrin proteins expressed in E. coli. Truncated [G(2), CE, E] and full-length [Ge(2)] forms of gephyrin are named according to the domain structure of the full-length molecule [variant (2,6)] (Prior et al., 1992). Cassette C2 is indicated by the black box. B, Pull-down assay. Full-length Ge(2) and truncated proteins were incubated with rat spinal cord extract as described in Materials and Methods. Bound proteins were separated by SDS-PAGE and visualized by silver staining. A prominent protein band migrating at 73 kDa (*) in lanes Ge(2) and G(2) was identified by mass spectrometry analysis as containing Hsc70 (two independent experiments; see supplemental Fig. 1, supplemental Table 1, available at www.jneurosci.org as supplemental material). C, Coimmunoprecipitation of gephyrin and Hsc70 from spinal cord extract from homozygous gephyrin-mRFP knock-in mice. Using beads conjugated with anti-T7 antibody, gephyrin complexed to Hsc70 and Pin1 was precipitated. Note that none of these proteins was precipitated from wild-type mouse spinal cord extract (two independent experiments). D, Rod structure of tagged-gephyrin [C2mRFP.Ge(2)] that contains mRFP and T7 tags in the middle of the C2 cassette (black box), of its G-domain, and the control construct (T7-mRFP). E, Endogenous Hsc70 coimmunoprecipitates with transfected C2mRFP.Ge(2). COS-7 cells were transfected with either C2mRFP.Ge(2) (left) or T7-mRFP (control, right) and subjected to coimmunoprecipitation with anti-T7 antibodies. Hsc70 was detected by Western blot analysis when recombinant gephyrin was expressed (two independent experiments).