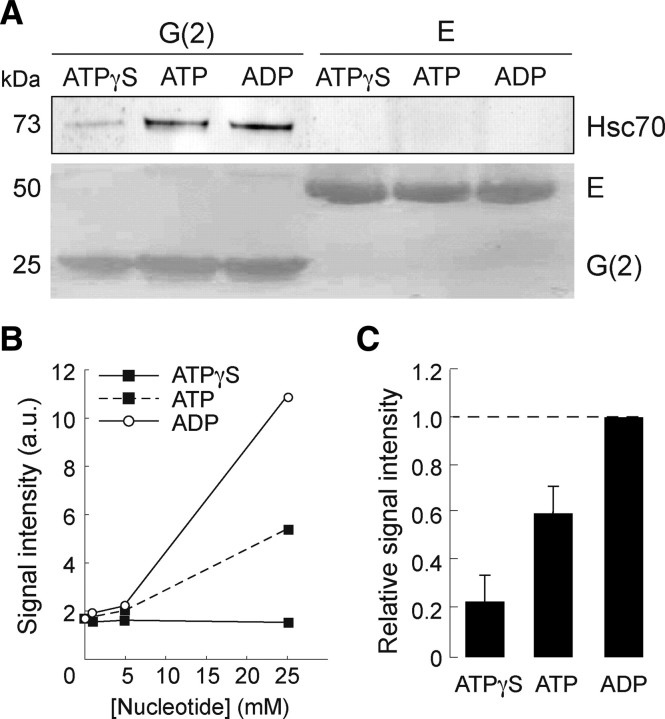
Figure 3.
Hsc70 interacts directly with the G-domain of gephyrin in an ADP-dependent manner. A, Purified recombinant Hsc70 was incubated with immobilized G(2)- or E-domains of gephyrin in the presence of 5 mm ATPγS, ATP or ADP, as described in Materials and Methods. Bound Hsc70 was detected after by Western blotting. Direct interaction of Hsc70 was obtained only with the G-domain (ECL+ detection, top). B, Binding of Hsc70 recovered after capture by the G(2)-domain in a similar experiment using nucleotide concentrations ranging from 0 to 25 mm. No concentration-dependent effect of ATPγS was observed above the basal interaction of the two proteins obtained in the absence of nucleotide. C, Nucleotide effect (5 mm final concentration) normalized to that of ADP. Data were averaged from four independent experiments (mean ± SEM). The presence of ATP resulted in a moderate increase in Hsc70 binding to the G-domain.