Figure 1.
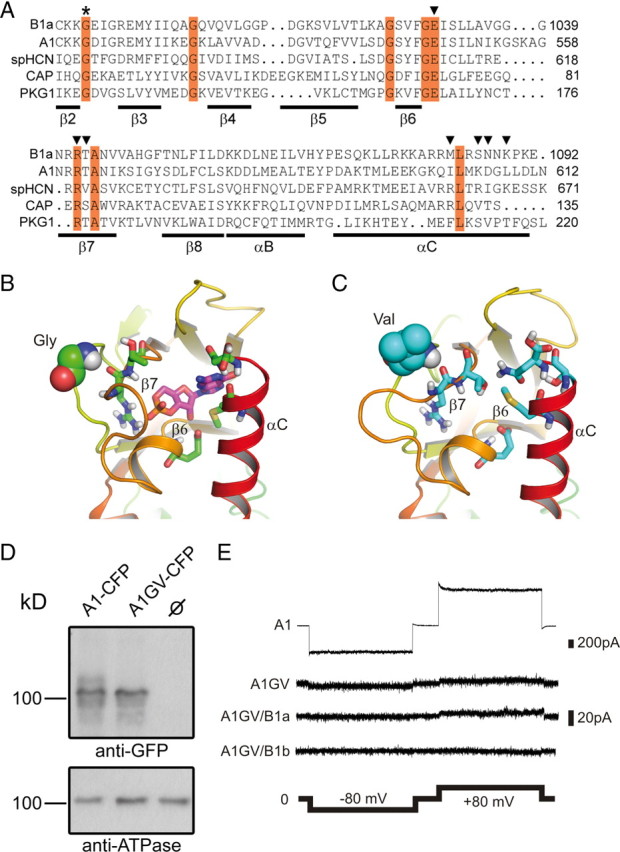
Effects of the GV mutation on the structure of the CNBD and cGMP-dependent channel activation. A, Sequence alignment of the β2–αC region of the CNBD of human CNGB1a, human CNGA1, sea urchin HCN1, Escherichia coli CAP, and human PKG1. Invariant residues are highlighted in red. The invariant glycine residue within the β2–β3 loop that is mutated to valine (GV mutation) in the CNGB1a subunit of RP patients is marked with an asterisk. Residues identified in the crystal structure of HCN channel CNBDs to participate in cyclic nucleotide-binding are marked with arrowheads (Zhou and Siegelbaum, 2007). B, C, Predicted structure of the cGMP binding pocket of CNGB1a (B) and CNGB1aGV (C). The sequences were threaded onto the crystal structure of the CNBD of spHCN (Flynn et al., 2007) by using MODELLER (Eswar et al., 2007) and analyzed using molecular dynamics simulations (see Materials and Methods). Only one monomer is shown for clarity. The glycine and the valine residue in the β2–β3 loop of CNGB1a and CNGB1aGV, respectively, are represented as space-filling spheres. Residues in the β6 and β7 strands as well as in the αC helix participating in cGMP binding are shown as sticks. In B, a cGMP molecule (shown as magenta sticks) was pasted onto the modeled structure based on information from the cGMP-bound spHCN CNBD crystal structure (Flynn et al., 2007). D, CNGA1GV is expressed at the plasma membrane of transfected HEK293 cells. Top, Western blot of membranes from HEK293 cells transfected with CNGA1-CFP or CNGA1GV-CFP, respectively. The blot was probed with anti-GFP antibody. Bottom, Membranes probed against the ATPase α subunit. CNBD, cyclic nucleotide-binding domain. E, Representative current traces elicited by 1 mm cGMP and voltage steps (2 s each) to −80 mV and +80 mV in excised patches from HEK293 cells expressing CNGA1 (A1) or CNGA1GV (A1GV), CNGA1GV/CNGB1a (A1GV/B1a), and CNGA1GV/CNGB1b (A1GV/B1b).
