Figure 2.
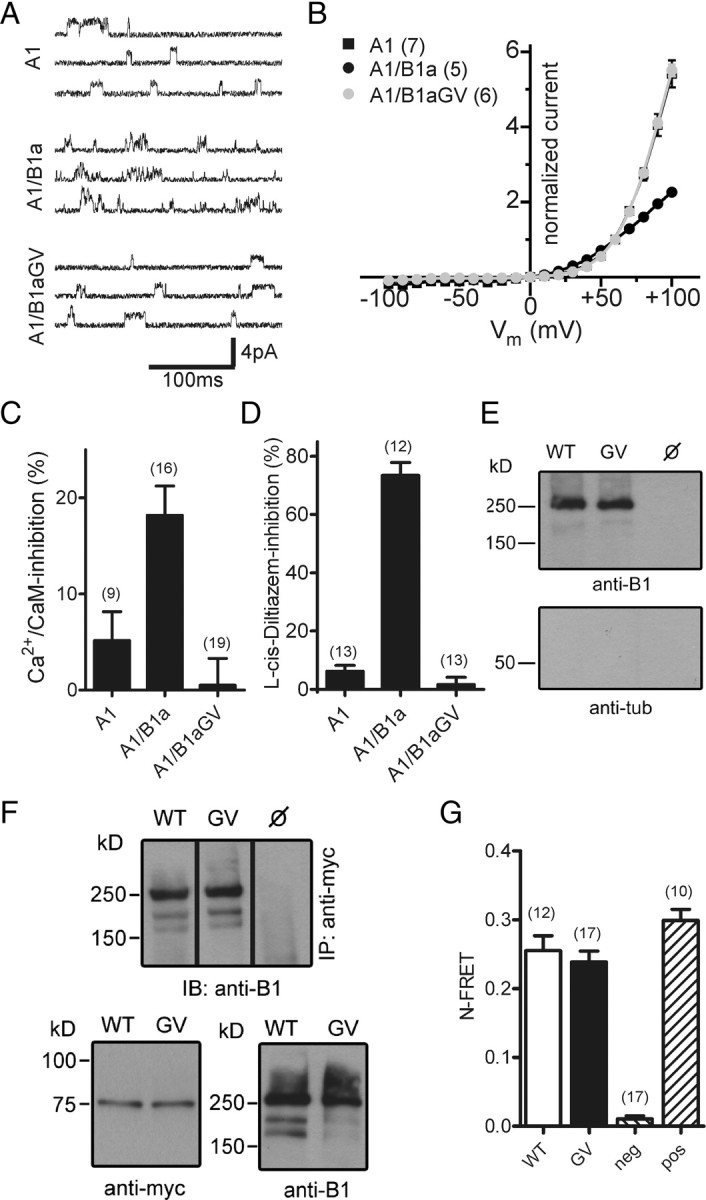
Heteromeric CNGA1/CNGB1aGV channels are present in the plasma membrane but are functionally inactive. A, Single-channel currents induced at +60 mV by 3 μm cGMP in excised patches of HEK293 cells transfected with CNGA1 (A1), CNGA1/CNGB1a (A1/B1a), or CNGA1/CNGB1aGV (A1/B1aGV). B, Current–voltage relation of A1, A1/B1a, and A1/B1aGV in the presence of 2 mm Ca2+ and 1 mm Mg2+ in the extracellular solution. Current was normalized to the current at +60 mV. C, Inhibition of A1, A1/B1a, and A1/B1aGV currents by 250 nm calmodulin/100 μm Ca2+ in the bath solution. Currents were induced at +80 mV by 100 μm cGMP. D, l-cis-Diltiazem (10 μm) block of A1, A1/B1a, and A1/B1aGV currents induced by 300 μm cGMP at +80 mV. E, Cell-surface biotinylation of nontransfected (ø) and CNGA1/CNGB1a- (WT) and CNGA1/CNGB1aGV- (GV) transfected HEK293 cells. Cells were biotinylated for 30 min at 4°C and probed with anti-B1 (top) or anti-tubulin (tub, bottom) antibodies. F, Coimmunoprecipitation from lysates of HEK293 cells cotransfected with myc-tagged CNGA1 and wild-type CNGB1a or CNGB1aGV, respectively. Top, Immunoprecipitation (IP) using anti-myc for pulldown and anti-B1 for detection. Bottom, Western blots showing the starting material for the co-IP probed with anti-myc (left) or anti-B1 antibodies (right). G, N-FRET ratios calculated from HEK293 cells cotransfected with CNGA1-CFP and YFP-CNGB1a (WT) or YFP-CNGB1aGV, respectively. HEK293 cells transfected with CFP and YFP-CNGB1a or a CFP-YFP tandem were used for the calculation of negative (neg) and positive (pos) control N-FRET values, respectively. The number of experiments is given in parentheses. All values are given as mean ± SE.
