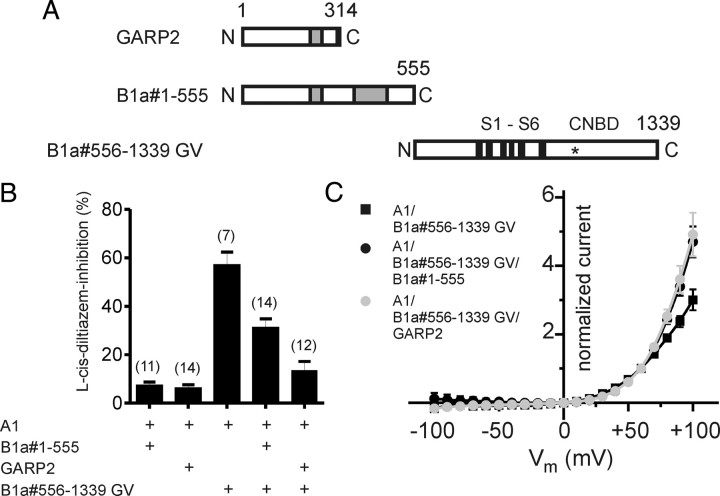
Figure 4.
GARP2 and the GARP domain of CNGB1a act as autonomous channel inhibitors. A, Schematic representation of GARP2, the N terminus of CNGB1a containing the complete GARP domain (B1a#1-555), and the truncated B1a#556-1339 GV channel. B, GARP2 and B1a#1-555 reduce the l-cis-diltiazem inhibition of A1/B1a#556-1339 GV channels. Shown is percentage inhibition by 10 μm l-cis-diltiazem of currents obtained after cotransfection of CNGA1 with various constructs as indicated. C, GARP2 and B1a#1-555 increase the outward rectification of A1/B1a#556-1339 GV currents in the presence of extracellular divalents. Shown is the current–voltage relation of A1/B1a#556-1339 GV, A1/B1a#556-1339 GV/GARP2, and A1/B1a#556-1339 GV/B1a#1-555 in the presence of 2 mm Ca2+ and 1 mm Mg2+ in the extracellular solution. Current was normalized to the current at +60 mV. The number of experiments is given in parentheses, and all values are given as mean ± SE.