Figure 7.
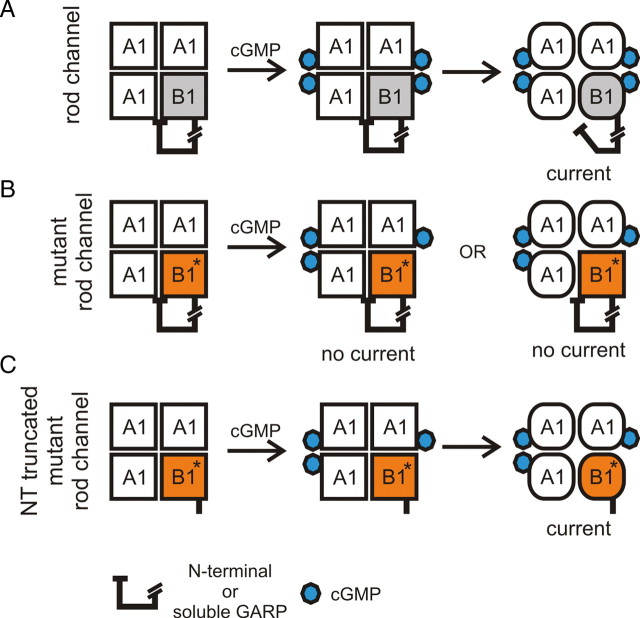
Model of the action of the GARP domain in wild-type (A) and mutant heteromeric CNG channels (B, C). A, In wild-type rod channels, GARP (N-terminal or soluble) serves as a gating inhibitor. Binding of cGMP to the CNGB1a subunit releases the tonic inhibition mediated by the GARP domain giving rise to channel opening. For reasons of clarity, the contemplable intermediates (i.e., if only one or two cGMP are bound to CNGA1) are not included in this model. B, The GV mutation in CNGB1a prevents cGMP binding to this subunit. Therefore, the tonic inhibition of GARP is not removed and the channels remain in the closed state. C, If GARP is not present the GV mutant heteromeric channels are able to open in the presence of cGMP.