Figure 7.
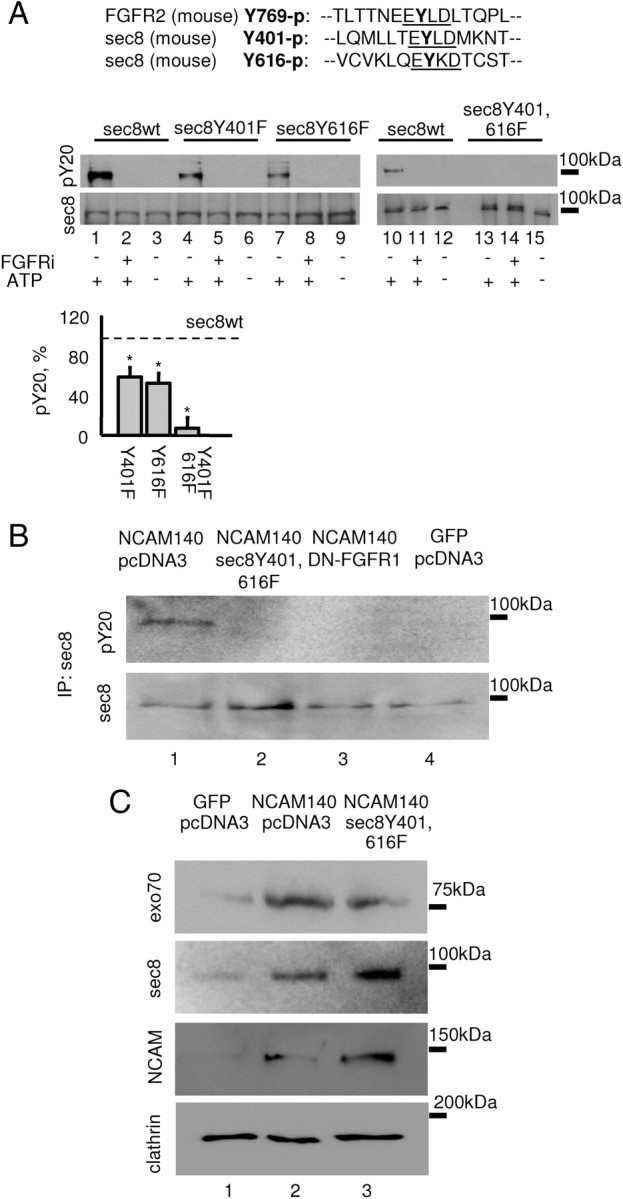
FGFR phosphorylates sec8 at the tyrosine residues Y401 and Y616. A, Tyrosine phosphorylation of sec8 analyzed by Western blot with sec8 and pY20 antibodies. Sec8 was incubated with the recombinant FGFR kinase domain in the presence or absence of ATP or FGFR inhibitor. Note that tyrosine phosphorylation of sec8 with mutated Y401 (lane 4) and Y616 (lane 7) is inhibited when compared to nonmutated sec8 (lane 1). Tyrosine phosphorylation of sec8Y401,616F is blocked (lane 13). A comparison of amino acid sequences surrounding the Y769 autophosphorylation site in FGFR2, and Y401 and Y616 in sec8 is shown at the top. Graph shows mean + SEM phosphorylation levels of sec8 mutants from three experiments normalized to sec8wt phosphorylation levels set to 100% (dashed line). *p < 0.05 (paired t test, compared to sec8wt levels). B, Sec8 immunoprecipitates from 3T3 fibroblasts transfected as indicated were analyzed by Western blot with sec8 and pY20 antibodies. Note that tyrosine phosphorylation of sec8 is increased in fibroblasts cotransfected with NCAM140 (lane 1) versus NCAM-negative fibroblasts (lane 4) and is inhibited in cells cotransfected with sec8Y401,616F (lane 2) and DN-FGFR1 (lane 3). C, Membranes isolated from 3T3 fibroblasts transfected as indicated were analyzed by Western blot with antibodies against sec8, exo70, and NCAM. Note that levels of exo70 and sec8 are increased in membranes from NCAM140 transfected (lane 2) versus NCAM-negative (lane 1) fibroblasts. Levels of membrane-associated sec8 are further increased in cells cotransfected with the sec8Y401,616F mutant (lane 3), which is also recognized by sec8 antibody (see A). Levels of membrane-associated exo70 are reduced in cells cotransfected with sec8Y401,616F. Labeling for clathrin served as loading control.
