Figure 3.
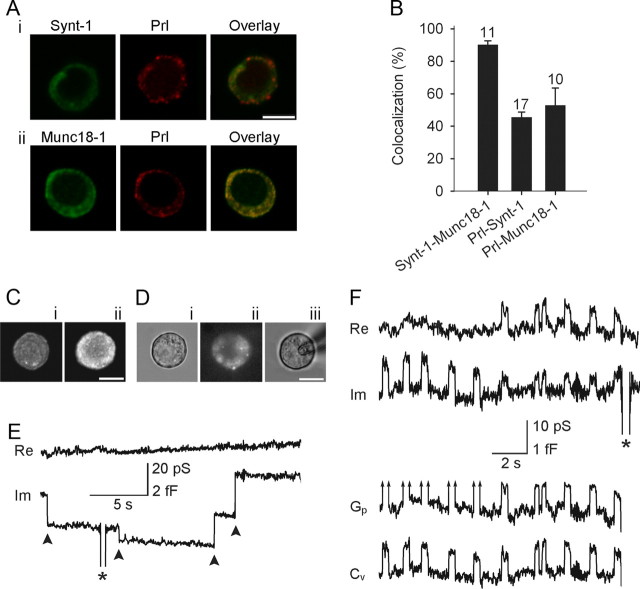
Discrete irreversible and reversible capacitance steps in lactotrophs transfected with Munc18-1. A, The colocalization of Synt1 (green) and Prl (red) (i), and Munc18-1 (green) and Prl (red) (ii). The colocalization of red and green channels is represented in yellow (overlay). B, The average colocalization of Munc18-1 with Synt1 was 90 ± 2% (with respect to Synt1). The colocalization of Prl with Synt1 and Munc18-1 was 46 ± 3% and 53 ± 11%, respectively (both with respect to Prl). Numbers above the error bars indicate the number of fluorescent cells analyzed. The colocalization between Prl-Synt1 and Prl-Munc18-1 was not significantly different. C, Confocal fluorescent micrographs showing the immunochemical localization of anti-Munc18-1 antibody in control cells (i) and in cells overexpressing WT Munc18-1 (ii). Note the increased fluorescence intensity of transfected lactotroph compared with the wild-type cell. Di, A DIC image of the cell. Successfully transfected lactotrophs were identified by cotransfecting respective Munc18-1 mutants with EGFP as shown in Dii, where the same cell is viewed in epifluorescence. Diii, The same cell as in Di and Dii in the cell-attached configuration. E, Representative irreversible downward and upward steps (arrowheads) in the Im trace, likely reflecting irreversible events of endocytosis and exocytosis, respectively. F, Representative reversible steps, observed in the Im trace, were used to calculate vesicle capacitance (Cv) and, if exhibiting a measurable projection to the Re trace (reversible steps on the right side of the panel), fusion pore conductance (Gp). Arrows in Gp traces indicate immeasurable projections. Asterisks denote calibration pulses. Scale bars, 10 μm.