Figure 8.
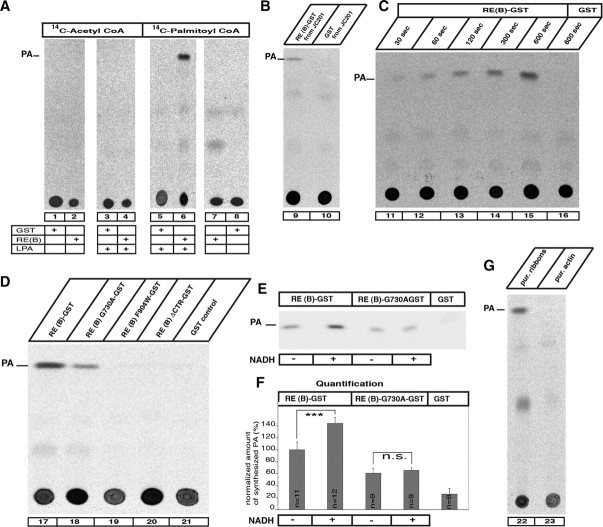
Biochemical analysis of RIBEYE's LPAAT activity. Reaction mixes containing radioactive acyl-CoAs, LPA, and the respective RIBEYE fusion and control proteins were analyzed by TLC for the presence of PA, the end product of LPAAT activity. A, RIBEYE generates PA from LPA if palmitoyl-CoA is present as activated fatty acid donor (lane 6), but not if other acyl-CoAs are used (lane 4). GST alone does not generate PA (lanes 1, 5, 8, 16, 21). B, RE(B)-GST purified from JC201 bacteria also displayed LPAAT activity (lane 9), indicating that the LPAAT activity is not due to contaminating bacterial protein with LPAAT activity. C, The LPAAT activity of RIBEYE is fast and is continuously generating PA (lanes 11–15). D, Wild-type RIBEYE(B)-GST fusion protein as well as RIBEYE(B)G730A-GST fusion protein demonstrated LPAAT activity (lanes 17, 18), whereas the RIBEYE point and deletion mutants RIBEYE(B)F904W-GST and RIBEYE(B)ΔCTR-GST as well as GST did not (lanes 19–21). E, Addition of NADH (200 nm) enhanced the LPAAT activity of RIBEYE(B)-GST but not the LPAAT activity of the NAD(H)-binding-deficient point mutant RIBEYE(B)G730A-GST. F, Quantification of LPAAT activity. NADH addition increased LPAAT activity of RIBEYE(B) wild-type protein by 39% with no effect on RIBEYE(B)G730A. Equimolar concentrations of fusion proteins were used in all experiments (140 nm). G, Purified synaptic ribbons (Schmitz et al. 2000) showed LPAAT activity, whereas purified actin did not (lanes 22, 23). ***p < 0.001. n.s., Nonsignificant.