Figure 9.
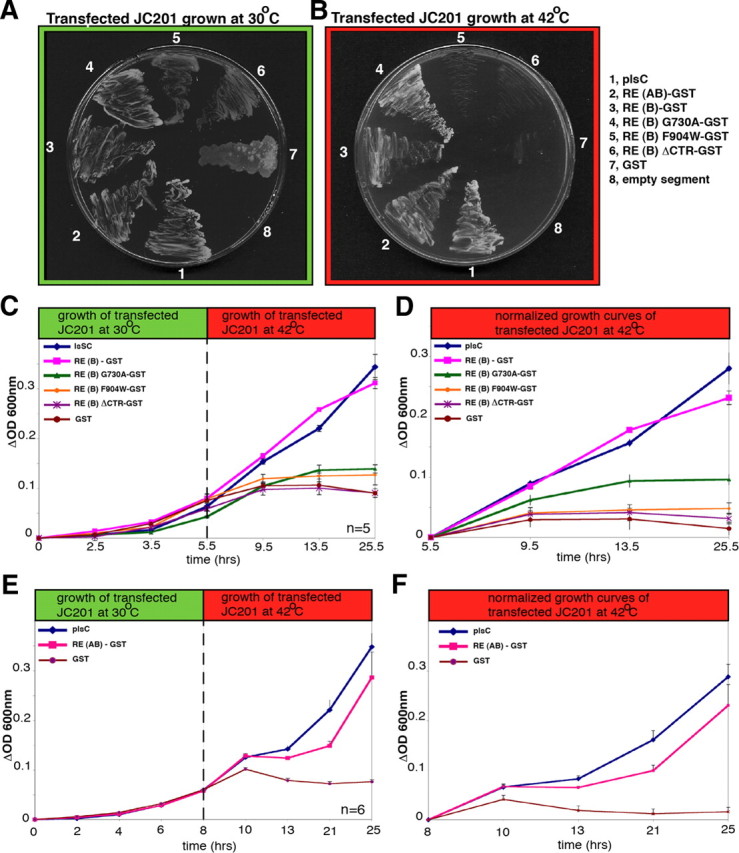
RIBEYE rescues LPAAT deficiency in JC201 bacteria. A, B, LPAAT complementation of transformed JC201 bacteria was qualitatively analyzed by growth of the respective bacteria at 42°C (on ampicillin/tetracyclin plates). Full-length RIBEYE(AB), RIBEYE(B), and RIBEYE(B)G730A complemented LPAAT deficiency, as evidenced by growth of the respective bacteria at 42°C, whereas RIBEYE(B)F904W, RIBEYE(B)ΔCTR, and GST did not. JC201 bacteria transformed with the latter RIBEYE mutants grew only at 30°C, but not at 42°C. plsC, encoding the endogenous bacterial LPAAT, served as a positive control (Coleman, 1990); an empty pGEX vector served as a negative control. C–F, LPAAT complementation of transformed JC201 bacteria was quantitatively analyzed by recording growth curves at 30°C (permissive temperature) and 42°C (restrictive temperature). plsC served as a positive control; an empty pGEX vector served as a negative control. Full-length RIBEYE(AB), RIBEYE(B), and RIBEYE(B)G730A complemented LPAAT deficiency, whereas RIBEYE(B)F904W and RIBEYE(B)ΔCTR did not complement LPAAT activity. Complementation of LPAAT deficiency by the eukaryotic RIBEYE protein was nearly as strong as LPAAT complementation by the genuine bacterial LPAAT protein encoded by plsC. n = 5 (C, D); n = 6 (E, F). Error bars indicate SEM.
