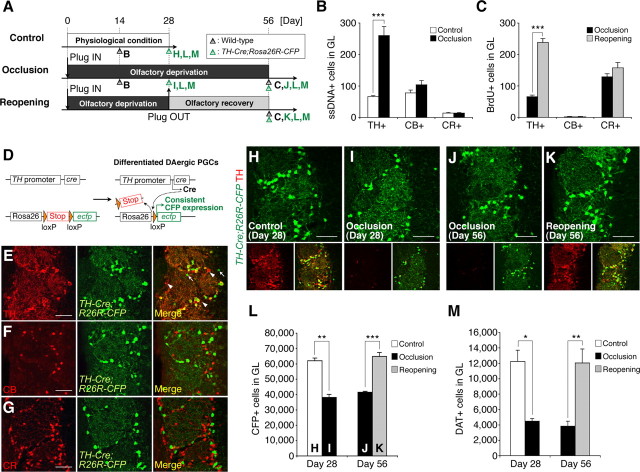
Figure 3.
Olfactory-input-dependent regulation on the survival of DAergic subtype of PGCs. A, Experimental design. Bold letters indicate the time points of the analyses shown in B, C, and H–M. B, C, Apoptosis (B) and the addition (C) of PGCs of each subtype upon naris occlusion and reopening. Naris occlusion and reopening significantly increased the number of apoptotic (ssDNA+) and newly added (BrdU+) TH+ DAergic PGCs, respectively, but not CB+ or CR+ PGCs (***p < 0.000005). BrdU was injected on day 28 (C). D, Diagram of the Cre-mediated recombination in TH+ DAergic PGCs used in this study. E–G, Coronal sections of the olfactory glomerular layer in TH-Cre;Rosa26R-CFP mice stained for TH, CB, and CR (red). The majority of the CFP+ PGCs (green) expressed TH. Only a small number of TH-/CFP+ PGCs and CFP-/TH+ PGCs (white arrows and arrowheads, respectively) were observed (E). The CB+ and CR+ PGCs never expressed CFP (F and G, respectively). See also Table 1. H–M, Olfactory-input-dependent dynamic alterations in the number of DAergic PGCs. Coronal sections of the olfactory glomerular layer in TH-Cre;Rosa26R-CFP mice (H–K) stained for GFP (green) and TH (red). The merged image (bottom right) shows that almost all the CFP+ DAergic PGCs also expressed TH in the control OB (H). Although the TH expression in these cells was mostly abolished by naris occlusion (day 28, I and day 56, J, red), these cells could be clearly detected by their stable and continuous CFP expression (green). The number of CFP+ DAergic PGCs was significantly reduced by 4 week naris occlusion (L, day 28, **p < 0.01), and increased by naris reopening (L, day 56, ***p < 0.0005), to a level comparable to that of the control OB (H). The number of DAT+ DAergic PGCs was also decreased by the 4 week naris occlusion (M, day 28, *p < 0.05) and increased by naris reopening (M, day 56, **p < 0.01). Scale bars: 50 μm. The data are presented as the mean ± SEM.