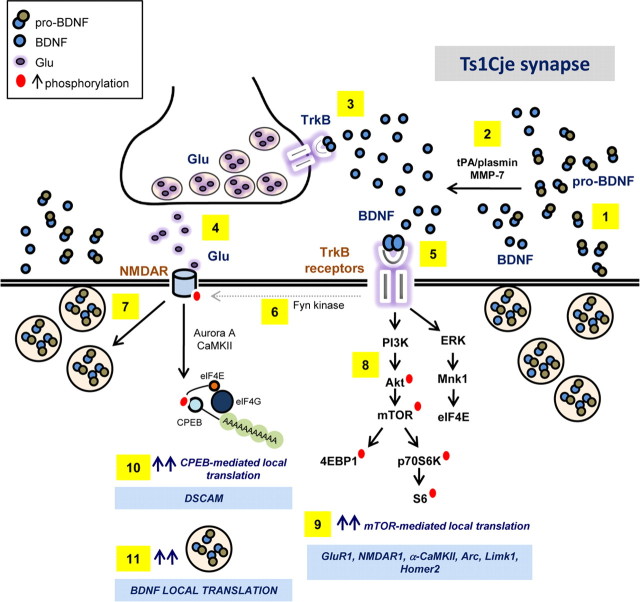
Figure 9.
Model proposed to explain why BDNF fails to regulate local translation in the hippocampus of Ts1Cje mice. Dendritic release of pro-BDNF and BDNF is increased in Ts1Cje hippocampal neurons (1). pro-BDNF is converted to mature BDNF extracellularly by tPA/plasmin, MMP-7 and other extracellular proteases (Waterhouse and Xu, 2009) (2). BDNF acts presynaptically at TrkB receptors (3) to enhance glutamate release (4) (Lessmann et al., 1994; Jovanovic et al., 2000). BDNF acts postsynaptically at TrkB receptors (5), promoting the phosphorylation of NMDAR subunits (6) (Suen et al., 1997; Lin et al., 1998), increasing the probability of NMDA channel opening (Jarvis et al., 1997; Levine et al., 1998) and, hence, increasing excitatory activity. Exacerbated glutamatergic activity provokes increased dendritic release of pro-BDNF and BDNF (7) (Korte, 2008; Tanaka et al., 2008). BDNF binding to postsynaptic TrkB receptors (5) also results in hyperactivation of the PI3K–Akt–mTOR signaling cascade (8), leading to increased local translation of dendritic mRNAs (9) like GluR1(which contributes to the increase in postsynaptic excitatory activity) and NMDAR1 (Schratt et al., 2004). Increased NMDAR-mediated neuronal activity in Ts1Cje hippocampal neurons also enhances CPEB1-mediated local translation of dendritic mRNAs such as DSCAM (10) (Alves-Sampaio et al., 2010). As BDNF mRNA is localized to dendrites (Tongiorgi et al., 1997; An et al., 2008), and its expression is regulated by CPEB1 (Oe and Yoneda, 2010) and a PI3K-dependent pathway (Righi et al., 2000), its local dendritic translation may also increase in trisomic mice, contributing to the overexpression of BDNF in dendrites of the Ts1Cje hippocampus (11). This glutamatergic positive-feedback loop may explain why hippocampal Ts1Cje synapses lose their sensitivity to further increases in BDNF-mediated activity (Alves-Sampaio et al., 2010; data herein) and attain a saturated state that impairs the modulation of synaptic plasticity.