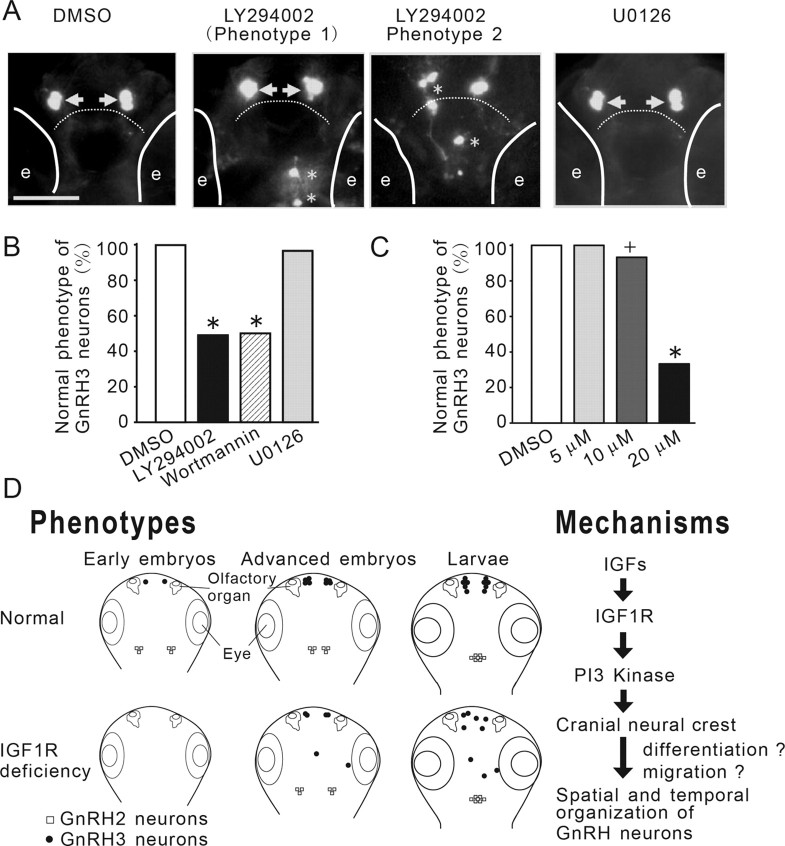
Figure 7.
The IGF action on GnRH3 neurons is mediated through the PI3K, but not the MAPK, signaling pathway. A, Inhibition of PI3K, but not MAPK, phenocopies IGF-deficient embryos. Tg(GnRH3:EGFP) embryos were treated with DMSO or the indicated inhibitors. GnRH3 neurons were visualized at 3 dpf. GnRH3 neurons at the two normal locations are indicated by arrows. Aberrant GnRH3 neurons are indicated by asterisks. Solid lines indicate the locations of eyes (e). Dashed line denotes the boundary between the olfactory bulb and the telencephalon. B, Quantitative results of A. n = 29∼37, +p < 0.05 and *p < 0.001 compared with the DMSO group. C, Dose-dependent effect of LY294002. D, A proposed model on the roles and underlying mechanisms of IGF signaling in regulating the temporal and spatial organization of newborn GnRH neurons in zebrafish embryos. Inhibition of IGF signaling delays the timing of GnRH2 and GnRH3 neuron development in early embryos and slows down the migration of GnRH2 neurons from lateral to the midline in advanced embryos. Inhibition of IGF signaling in early embryonic stage also results in abnormal distribution of GnRH3 neurons in the larval brain. Right, IGFs activate the IGF1R-PI3K pathway to regulate the migration/differentiation of cranial neural crest cells in early embryogenesis. This in turn results in altered temporal and spatial organization of newborn GnRH3 neurons.