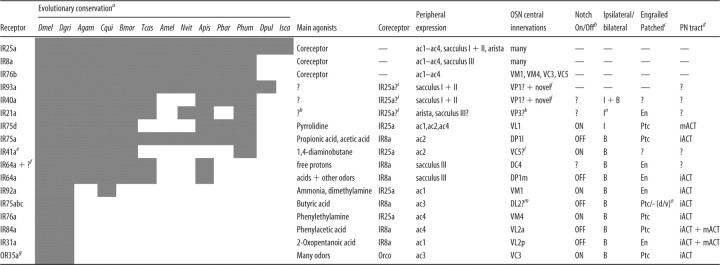
Table 1.
Pharmacological, molecular, evolutionary, and anatomical properties of the IR olfactory pathways
aSensory channels are ordered by the approximate time of their evolution, as predicted by the conservation across Protostomia of the receptors they express. Data for the sole member of the OR family expressed in coeloconic sensilla, OR35a, are also shown (Yao et al., 2005). Unless otherwise indicated, all data come from this work or from Benton et al. (2009). Presence of putative receptor orthologs in a species is indicated by a shaded cell. Bioinformatic data is taken from Croset et al. (2010) and Smith et al. (2011). Dmel, Drosophila melanogaster; Dgri, Drosophila grimshawi; Agam, Anopheles gambiae; Cqui, Culex quinquefasciatus; Bmor, Bombyx mori; Tcas, Tribolium castaneum; Amel, Apis mellifera; Nvit, Nasonia vitripennis; Apis, Acyrthosiphon pisum; Pbar, Pogonomyrmex barbatus; Phum, Pediculus humanus humanus; Dpul, Daphnia pulex; Isca, Ixodes scapularis. Note that the absence of certain genes in inner insect lineages (e.g., AmelIR40a and NvitIR40a) may reflect either loss of these genes or simply gaps in the available genome sequence.
bData from Endo et al. (2007).
cData from Chou et al. (2010a).
dData from Marin et al. (2002), Wong et al. (2002), Jefferis et al. (2007), and Chiang et al. (2011). PNs arising from anterodorsal and lateral neuroblasts project their axons via the inner antennocerebral tract (iACT), sending collaterals to the mushroom body calyx and terminating in the lateral horn. Axons of PNs arising from the ventral neuroblast project via the middle antennocerebral tract (mACT) directly to the lateral horn, bypassing the mushroom body completely (Fig. 6A).
eIR41a was not detected by RNA in situ hybridization in our previous analysis (Benton et al., 2009), but reannotation of the gene structure (Croset et al., 2010) and subsequent RNA probe redesign revealed coexpression of IR41a with IR76b in ac2 neurons (data not shown).
fIR64a is necessary for responses of DC4 neurons to acid stimuli (free protons) but is not sufficient to confer acid responses when misexpressed (Ai et al., 2010), suggesting the existence of an additional receptor.
gData from Couto et al. (2005), Fishilevich and Vosshall (2005), and Yao et al. (2005).
hAristal neurons have been shown to function as thermosensors (Gallio et al., 2011), but the role of IR21a in mediating physiological responses to temperature changes, if any, is unknown.
iThe coreceptor IR25a, but not IR8a, is robustly expressed in these populations of neurons (Benton et al., 2009), suggesting that it may act as a coreceptor for these sacculus and aristal IRs.
jVP1 was originally described, through cobalt backfill analysis, as one of the five glomeruli receiving unilateral input located between VP2 and VP3 (Stocker et al., 1983). The complete morphology of this glomerulus has not been reported, however, and the inability to visualize this neuropil with nc82 staining has led to subsequent antennal lobe maps either excluding VP1 (Laissue et al., 1999; Couto et al., 2005) or presenting different representations of its position (Chou et al., 2010a; Yu et al., 2010a). The location and unilateral innervation of the column part of the IR40a axonal arbors in the antennal lobe suggests that a subset of these neurons could correspond to the “VP1 neurons” described by Stocker et al. (1983) but that IR40a-expressing OSNs comprise a heterogeneous population with more extensive and novel innervations in the antennal lobe. Although a faithful GAL4 reporter for IR93a is not available, coexpression of endogenous IR93a and IR40a in the sacculus (Benton et al., 2009) allows us to infer the central projections of IR93a neurons.
kAristal neurons were shown to innervate VP2 and VP3 glomeruli by cobalt backfill analysis (Stocker et al., 1983). A GAL4 reporter for GR28b.d is expressed in a subpopulation of aristal neurons and innervates the medial VP2 glomerulus (Thorne and Amrein, 2008). We found that IR21a mRNA is expressed in the complementary set of aristal neurons to this reporter line (data not shown), suggesting that IR21a neurons correspond to those innervating the lateral VP3 glomerulus.
lIR41a projections may correspond to the novel glomerulus described by Endo et al. (2007).
mThe innervation of IR75a/IR75b/IR75c ac3 neurons was deduced by correlation of peripheral electrophysiological and central optical imaging data (Figs. 1, 4) but could not be verified by a specific GAL4 line.
nIpsilateral projections of aristal neurons were demonstrated in cobalt backfill analysis (Stocker et al., 1983).
oSeveral analyses divide the DL2 glomerulus morphologically into dorsal (d) and ventral (v) compartments (Laissue et al., 1999), although their functional distinction, if any, is unknown. ? and — indicate when data are unavailable or inapplicable, respectively.