Abstract
Motor behavior is generated by specific neural circuits. Those producing locomotion are located in the spinal cord, and their activation depends on descending inputs from the brain or on sensory inputs. In this study, we have used an in vitro brainstem-spinal cord preparation from adult zebrafish to localize a region where stimulation of descending inputs can induce sustained locomotor activity. We show that a brief stimulation of descending inputs at the junction between the brainstem and spinal cord induces long-lasting swimming activity. The swimming frequencies induced are remarkably similar to those observed in freely moving adult fish, arguing that the induced locomotor episode is highly physiological. The motor pattern is mediated by activation of ionotropic glutamate and glycine receptors in the spinal cord and is not the result of synaptic interactions between neurons at the site of the stimulation in the brainstem. We also compared the activity of motoneurons during locomotor activity induced by electrical stimulation of descending inputs and by exogenously applied NMDA. Prolonged NMDA application changes the shape of the synaptic drive and action potentials in motoneurons. When escape activity occurs, the swimming activity in the intact zebrafish was interrupted and some of the motoneurons involved became inhibited in vitro. Thus, the descending inputs seem to act as a switch to turn on the activity of the spinal locomotor network in the caudal spinal cord. We propose that recurrent synaptic activity within the spinal locomotor circuits can transform a brief input into a well coordinated and long-lasting swimming pattern.
Introduction
Microcircuits in the CNS control different motor behaviors (Marder et al., 2005; Kiehn, 2006; Goulding, 2009; Grillner and Jessell, 2009; El Manira and Kyriakatos, 2010). The spinal cord can generate different motor behaviors through coordination of the activity of local microcircuits (Grillner, 2003, 2006; Kiehn, 2006; Berkowitz et al., 2010; Fetcho and McLean, 2010). Each of these behaviors can vary in pattern, speed, and force (e.g., activation and coordination of segmental muscles during escape and swimming in fish or coordination of limb movements during walking, trot, gallop, and scratch in legged animals). Thereby, some of the neurons can be dedicated to a specific motor microcircuit underlying a given behavior, while others are shared by different microcircuits to mediate different behaviors (El Manira and Clarac, 1994; Svoboda and Fetcho, 1996; Grillner, 2006; Grillner and Jessell, 2009; Berkowitz et al., 2010; Fetcho and McLean, 2010; Roberts et al., 2010).
Spinal cord in vitro preparations from different animals have been used to study locomotor circuits (Drapeau et al., 2002; Grillner, 2003; Kiehn, 2006; Fetcho et al., 2008; Grillner and Jessell, 2009; Roberts et al., 2010). In most of these preparations, the induction of a motor pattern relies mostly on the use of excitatory amino acid agonists in combination with modulatory transmitters (Schmidt et al., 1998; Clarac et al., 2004; LeBeau et al., 2005; Kiehn, 2006; McDearmid and Drapeau, 2006; Taccola and Nistri, 2006; Gabriel et al., 2008, 2009). However, a prolonged application of transmitter agonists can potentially change the properties of circuit neurons. To overcome this concern, in some preparations spinal circuits are activated by continuous stimulation of descending inputs with the resulting locomotor activity declining rapidly after termination of the stimulation (Fetcho and Svoboda, 1993; El Manira et al., 1997; Cabelguen et al., 2003; Dubuc et al., 2008; Jordan et al., 2008). However, the use of constant stimulation precludes separation of the mechanisms intrinsic to the spinal circuits from those dependent on descending inputs. The role of descending inputs is unclear as to whether they only provide a “go” signal (Deliagina et al., 2002; Dubuc et al., 2008; Jordan et al., 2008) or whether the descending neurons are involved in the generation of the locomotor rhythm (Chong and Drapeau, 2007; Roberts et al., 2008, 2010; Soffe et al., 2009).
In this study, we sought to determine the role of descending inputs in the generation of locomotor activity in an in vitro brainstem-spinal cord preparation of adult zebrafish. We show that a transient stimulation of descending inputs induces sustained swimming activity. The pattern and frequency of the induced activity are comparable with those of intact animals. The firing pattern of motoneurons and the underlying membrane potential oscillations showed differences depending on whether the swimming activity was induced by stimulation of descending inputs or by activation of NMDA receptors. Finally, during escape the swimming was interrupted and some recruited motoneurons became inhibited. Thus, our results indicate that descending excitatory inputs act only as a trigger to initiate the activity of local spinal circuits generating swimming.
Materials and Methods
In vitro spinal cord preparation.
Zebrafish (ABC and AB/Tuebingen strains) were raised and kept according to established procedures. The animals used in this study were 8–15 weeks old (body length 18–30 mm). All experimental protocols were approved by the Animal Research Ethical Committee, Stockholm. Zebrafish were cold anesthetized in a slush of frozen fish saline (134 mm NaCl, 2.9 mm KCl, 2.1 mm CaCl2, 1.2 mm MgCl2, 10 mm HEPES, and 10 mm glucose, pH 7.8, with NaOH, 290 mOsm) and eviscerated. The skull was opened, and the brain was cut at the level of the midbrain. The epaxial musculature was removed up to the caudal end of the dorsal fin, leaving the musculature at the tail intact. Here, the skin was gently removed to reveal the underlying myotomes. To record locomotor activity, glass suction electrodes were positioned over the intermyotomal clefts wherein the axons of motoneurons lie. After removal of the vertebral arches, the entire spinal cord together with the hindbrain and the caudal musculature were then cut out, together with the vertebral column (see Fig. 1A). For in vitro swimming experiments, the spinal cord with brainstem was fixed dorsal side up with Vaseline. For split bath experiments, low-melting agar (0.1%) was used and care was taken to prevent solution leaking from the rostral to the caudal pool and vice versa. For intracellular recordings, the preparation was transferred to a recording chamber, and placed on the side and fixed with Vaseline. The preparation was perfused with d-tubocurarine to effectively block neuromuscular junctions and to abolish muscle twitches for the duration of the experiment. The effect of d-tubocurarine was considered total if no muscle activity was detected, and motor activity was recorded only when the extracellular electrodes were placed over the intermyotomal clefts. During experiments, the preparations were continuously perfused with oxygenated fish saline at 20–22°C.
Figure 1.
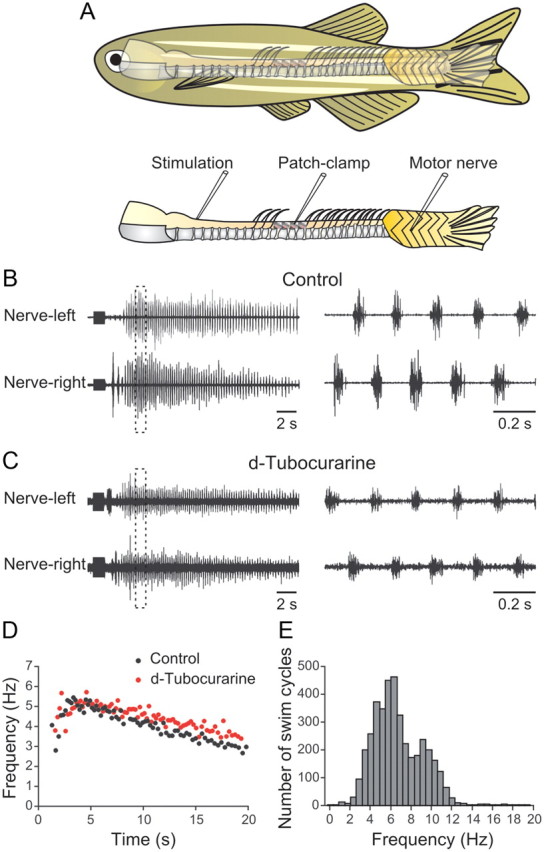
A, Experimental setup showing the placement of electrical stimulation, patch-clamp, and motor nerve recording electrodes. The position of the exposed part of the spinal cord is shown in relation to the whole body of the zebrafish. B, Stimulation (1 s, 40 Hz) of the spinal cord at the junction with the brainstem induces a long episode of swimming activity. C, Blockade of synaptic transmission at the neuromuscular junction by d-tubocurarine (10 μm) decreases the amplitude of the locomotor bursts (traces are magnified four times compared with the control traces in B). D, Graph showing the instantaneous burst frequency during one swimming episode in control and in d-tubocurarine. E, Graph showing the distribution of the instantaneous burst frequency. The data are pooled from 24 different preparations.
Electrophysiology.
Extracellular recording and stimulation electrodes were pulled from borosilicate glass (1 mm outer diameter, 0.87 mm inner diameter; Harvard Apparatus) on a Flaming/Brown microelectrode puller (P-87, Sutter Instruments), broken down to the desired tip diameter (15–25 μm) and fire polished. Extracellular recordings were performed from the motor nerves running through the intermyotomal clefts at the tail, where the musculature was left intact. Another electrode was placed dorsally on the rostral spinal cord to elicit locomotor episodes by electrical stimulation. Extracellular signals were amplified (gain: 10,000) with a differential AC amplifier (AM Systems) and filtered with low and high cutoff frequencies of 300 Hz and 1 kHz, respectively. Intracellular whole-cell recordings were performed from identified spinal motoneurons rostral to the peripheral nerve recording. For intracellular recordings, electrodes were pulled from borosilicate glass (1.5 mm outer diameter, 0.87 mm inner diameter) on a vertical puller (Narishige) and filled with intracellular solution (120 mm potassium gluconate, 5 mm KCl, 10 mm HEPES, 4 mm Mg2ATP, 0.3 mm Na4GTP, 10 mm sodium phosphocreatine, pH 7.4 with KOH, 275 mOsm), yielding resistances of 8–12 MΩ. Cells were visualized with a microscope (Axioskop FS Plus, Zeiss) equipped with infrared differential interference contrast optics and a CCD camera with frame grabber (Hamamatsu). Intracellular signals were amplified with a MultiClamp 700B intracellular amplifier (Molecular Devices) and low-pass filtered at 10 kHz. In current-clamp recordings, the bridge was always compensated and no bias current was injected. The following drugs were used in these experiments: AP-5, GYKI 52466, NMDA, and strychnine (Tocris Bioscience) and d-tubocurarine (Sigma-Aldrich).
In vivo recording of swimming activity.
Electromyogram (EMG) recordings were obtained with two varnish insulated steel wires (diameter: 46 μm). The wires were pulled through a hollow needle (Terumo Medical Corporation) and bent to form hooks at the needle's tip (Mentel et al., 2006). After the animals were anesthetized with 0.015% MS-222 (ethyl 3-aminobenzoate methane-sulfonate; 5 min), the hollow needle was inserted into the myotomal muscle 5 mm caudal and dorsal to the pectoral fin and retracted, leaving the wires in place. Zebrafish were prepared for bilateral EMG recording and then placed in an aquarium measuring 0.35 × 0.25 × 0.25 m. After recovery from anesthesia (15–20 min), animals could swim and produce spontaneous escape. In some instances, escape behavior was evoked by casting a shadow over the animal. Swimming sequences were monitored and recorded with a video camera (NV-VZ10, Panasonic). EMG signals were amplified by a custom-made AC amplifier (Elektronikwerkstatt, Cologne University) and bandpass filtered (200 Hz–3 kHz).
Data analysis.
In vivo data were digitized with a constant sampling rate of 12.5 kHz using an analog-to-digital converter (CED 1401 plus, Cambridge Electronic Design). The data were stored and analyzed by using CED Spike 2 software (version 5.14, Cambridge Electronic Design). For analysis only swim sequences during which animals showed straightforward swimming were selected. In vitro data were digitized at 10 kHz (extracellular recordings) or 20 kHz (patch recordings) with a Digidata 1322A A/D converter (Molecular Devices) and acquired on a personal computer using pClamp software (Molecular Devices). Data analysis was performed in Spike2 (version 7, Cambridge Electronic Design). Motor nerve bursts were rectified, smoothed (time constant: 0.01 s), and down-sampled to 1 kHz. The swimming period was determined by measuring the interval between two consecutive activity peaks. All values are given as mean ± SEM. The significance of differences of means between experimental groups and conditions was analyzed using Student's two-tailed t test. Means were considered statistically significant at p values of <0.05.
Results
Long-lasting swimming activity induced by stimulation of descending inputs
To be able to assess whether stimulation of descending inputs from the brainstem can elicit swimming activity, we developed an in vitro brainstem-spinal cord preparation of adult zebrafish (Fig. 1A). Muscles in the caudal part were left intact to be able to record motor nerve activity, and a part of the spinal cord rostral to the dorsal fin was exposed to enable whole-cell recordings from locomotor network neurons. A brief electrical stimulation of descending axons at the junction between the brainstem and spinal cord induced long-lasting swimming activity characterized by left-right alternation of muscle contractions (Fig. 1B). This locomotor activity persisted after blockade of neuromuscular junctions with d-tubocurarine (10 μm) (Fig. 1C). The locomotor frequency and burst amplitude were higher at the beginning of the swimming episode and declined gradually until the activity was terminated (Fig. 1D). The induced swimming frequency ranged from 1 to 17 Hz, peaking at 6 Hz (n = 24) (Fig. 1E), and the episode duration ranged from 10 to 200 s (n = 24). The burst frequency was not affected by d-tubocurarine, while the amplitude of the burst was decreased because of a blockade of muscle contraction (Fig. 1B–D). These results show that a brief stimulation of descending inputs from the brainstem is transformed into long-lasting swimming episodes in adult zebrafish.
Localization of the stimulation site and optimal parameters
The optimal location to induce coordinated swimming activity was determined by varying the position of the stimulation electrode in the caudal hindbrain and rostral spinal cord (Fig. 2A). Swimming activity could be reliably induced when the stimulation electrode was positioned between the first and second segment of the spinal cord, which correspond to the level of the third vertebral bone caudal to the Webberian apparatus (Fig. 2A, position 3, D). Stimulation of descending inputs either rostrally or caudally to this optimal position resulted in a tonic uncoordinated activity in the peripheral motor nerve (Fig. 2B,C,E). The stimulation electrode was always positioned on the midline. No bilateral rhythmic activity was obtained when the stimulation was delivered away from the midline to either side of the spinal cord. These results demonstrate the existence of an optimal region that, when briefly stimulated, induces long episodes of swimming activity.
Figure 2.
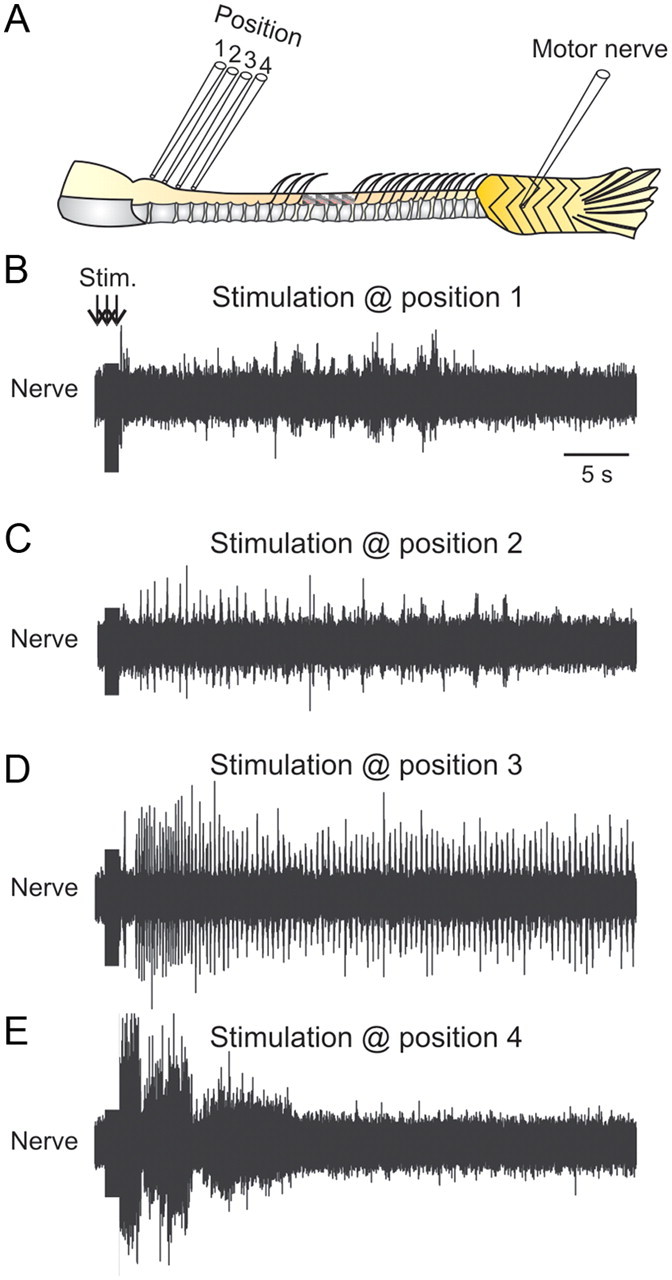
Localization of the optimal position to elicit swimming activity. A, Experimental setup showing the different positions tested to induce swimming activity. B, Stimulation of the rostral brainstem induces only tonic activity. C, Stimulation of the caudal brainstem produces a few bursts recorded in the peripheral motor nerve. D, Stimulation at the junction between the brainstem and spinal cord always induces long-lasting swimming activity. E, Moving the stimulation electrode to a more caudal position only induces tonic activity that does not develop into a swimming pattern.
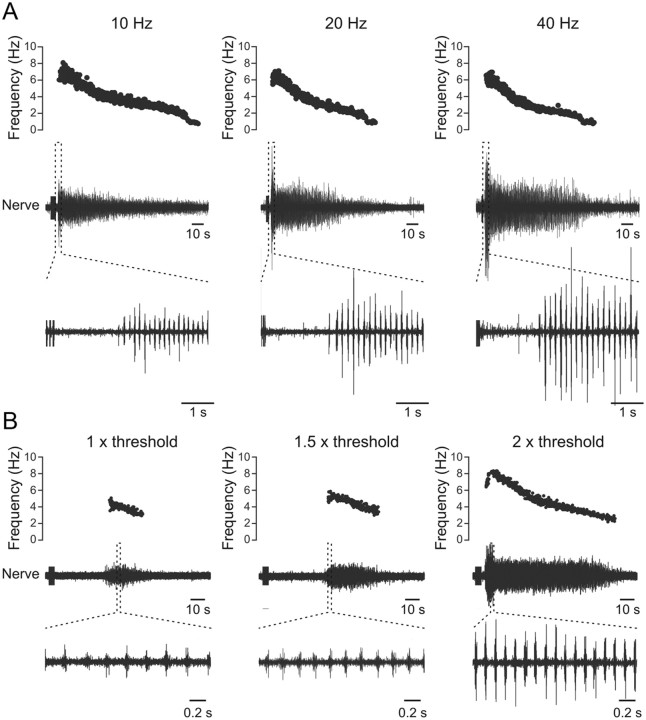
We also assessed the optimal stimulation parameters to elicit swimming activity. We first tested the effect of changing the frequency of stimulation. Swimming activity could be efficiently induced with electrical stimulation consisting of 10 pulses delivered at a frequency of 10–80 Hz. However, the more consistent swimming episodes were elicited with stimulation frequencies of 10–40 Hz (Fig. 3A). The delay to the onset of the swimming activity decreased while the amplitude of the locomotor burst increased when the stimulation frequency was increased from 10 to 20 and 40 Hz (Fig. 3A). Further increase in the stimulation frequency to 60 and 80 Hz only induced shorter swimming episodes with low burst frequency and high incidence of irregular bursts (data not shown). Second, we tested the influence of increasing the amplitude of the stimulation pulses while keeping their frequency constant at 40 Hz (Fig. 3B). The threshold for eliciting swimming activity was determined as the minimum stimulation amplitude that induced a bout of rhythmic bursts (Fig. 3B). Increasing the stimulation amplitude resulted in longer swimming episodes, larger burst amplitude, and shorter delay to onset of activity (Fig. 3B). These results indicate that specific stimulation parameters are required to reliably induce long episodes of swimming activity with appropriate burst frequency and amplitude. The generation of swimming activity is a graded response that may depend on appropriate supraspinal activity.
Figure 3.
Defining the optimal stimulation parameters to induce swimming in vitro. A, The latency to the start of the swimming activity is decreased while the amplitude of the bursts is increased as a function of increasing stimulus frequency from 10 to 20 and 40 Hz. B, Increasing the stimulus intensity increases the burst frequency and amplitude as well as the episode duration, while the delay to the onset of swimming decreases.
Swimming motor patterns and their frequency range in intact zebrafish
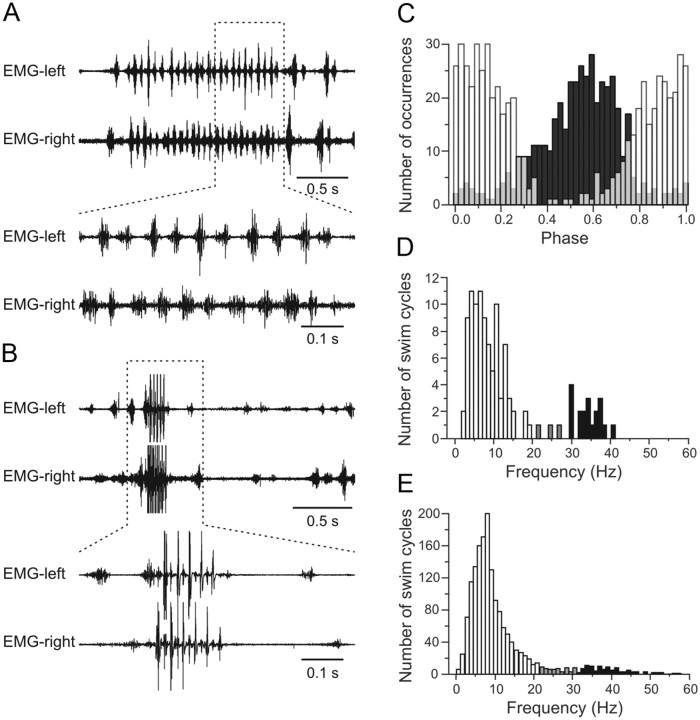
To examine how the frequencies of swimming activity induced in the in vitro preparation match those of the intact zebrafish, we recorded muscle activity in freely swimming animals. Bouts of swimming activity were recorded in intact animals with an alternating pattern of activity between left and right side muscles (n = 8) (Fig. 4A–C). Sometimes, the animals generated sequences of escape either spontaneously or upon casting a shadow on the animals (Fig. 4B). These sequences consisted of a few, in general three to five, short cycles of large EMG activity with clear alternation between left and right side muscles of the animals. Swimming activity appeared to be interrupted during escape, but often recovered gradually afterward (Fig. 4B). Swimming and escape activities were easily distinguishable (1) by their frequency and (2) by the amplitude of the EMG activity, which presumably involve different muscles fibers (Fig. 4B,D) (Liu and Westerfield, 1988). In individual animals, the frequencies of swimming activity ranged from 2 to 21 Hz peaking at ∼8–10 Hz (Fig. 4D, white bars; data from a single animal). The frequencies generated during escape were much faster, ranging from 30 to slightly >40 Hz (Fig. 4D, black bars). The small number of swimming cycles with frequencies between 21 and 30 Hz resulted from transitions from swimming to escape and vice versa (Fig. 4D, gray bars). The burst frequencies of swimming and escape motor activity were similar across animals (n = 8) (Fig. 4E). For swimming, they ranged again from ∼1 Hz to slightly >20 Hz, but never exceeding 21 Hz (n = 8) (Fig. 4E, white bars), and for escape from ∼30 Hz toward faster frequencies. We never observed motor activity during escape lower than 29 Hz (Fig. 4E, black bars). Across animals, the maximal frequency reached during escape was 52 Hz. These data show that the range of the swimming frequency generated in the in vitro preparation by electrical stimulation of descending axons falls well in the range of swimming frequencies generated in freely moving zebrafish.
Figure 4.
Swimming activity in intact zebrafish. A, Swimming activity is recorded in intact animals using wire electrodes inserted into muscles. EMG activity alternates between the left and right side of the body. B, Swimming activity is interrupted by escape movements that consist of large motor units with a higher burst frequency than swimming. C, Phase relationship between the activity of left (white bars) and right (black bars) EMGs displaying alternating pattern. D, Distribution of the burst frequency of swimming and escape activity in a single animal. E, Pooled data from all animals tested showing the distribution of the burst frequency during swimming and escape. In D and E, white bars represent swimming activity, black bars represent escape, and gray bars represent cycles of transition between escape and swimming.
Swimming activity is generated by the spinal locomotor network
In most preparations in which descending inputs were stimulated to induce locomotor activity, the stimulation was continuously delivered to maintain the bursting activity (Fetcho and Svoboda, 1993; Sirota et al., 2000; Cabelguen et al., 2003). In our study, the swimming episode persisted up to several minutes after the end of the stimulation of the descending inputs. It is thus possible that locomotor activity is the result of activation of a local hindbrain network of interneurons that then drives the spinal locomotor network (Li et al., 2006, 2010; Soffe et al., 2009; Li et al., 2010). To test for this, synaptic transmission was selectively blocked in the hindbrain and rostral spinal cord. In these experiments, the recording chamber was divided into two pools separated with an agar barrier to allow separate perfusion of the hindbrain together with the rostral spinal cord and the caudal spinal cord (Fig. 5A). In control conditions, stimulation of the rostral spinal cord induced a swimming episode consisting of clear bursts in the motor nerve (Fig. 5B). Blockade of chemical synaptic transmission in the hindbrain and rostral spinal cord using a Ca2+-free solution containing the ionotropic glutamate receptor antagonists AP-5 (50 μm) and GYKI 52466 (30 μm) had no effect either on the burst frequency or on the duration of the swimming episode (Fig. 5C). The change in the burst frequency during the swimming episode was similar in control and Ca2+-free solution containing ionotropic glutamate receptor antagonists (Fig. 5D). The peak burst frequency was 4.9 ± 0.5 Hz in control and 4.8 ± 0.6 Hz in Ca2+-free solution (p > 0.05; n = 8), while the duration of the swimming episode was 111.8 ± 30.0 s in control and 99.4 ± 29.5 in Ca2+-free solution (p > 0.05; n = 8). Thus, swimming activity induced by the focal and brief electrical stimulation seems to be generated at the level of the caudal spinal cord rather than the result of activating a local network of interneurons in the hindbrain or rostral spinal cord.
Figure 5.
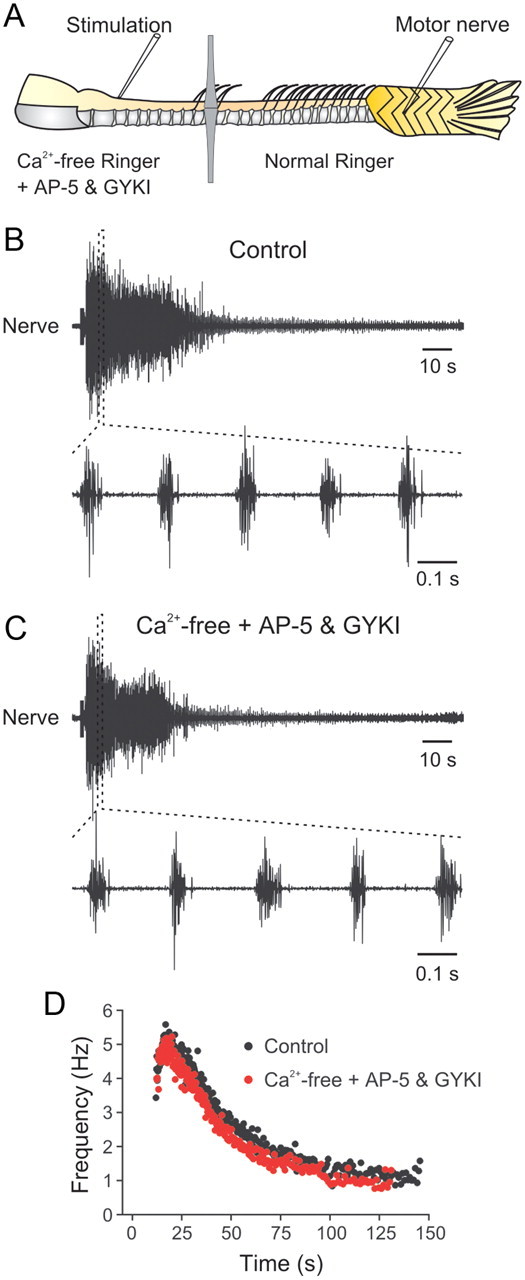
Swimming activity does not depend on synaptic activity between neurons at the site of stimulation. A, Experimental setup with the recording chamber subdivided into two pools that are perfused with physiological solutions of different compositions. B, In normal physiological solution in both pools, swimming activity is elicited by stimulation of the optimal position at the junction between the brainstem and spinal cord. C, Swimming activity can still be elicited when the rostral pool containing the brainstem and the rostral spinal cord is perfused with Ca2+-free solution containing AP-5 and GYKI 52466 to block chemical synaptic transmission and ionotropic glutamate receptors. D, Graph showing the instantaneous frequency during a swimming episode in control and in Ca2+-free solution with ionotropic glutamate receptor antagonists.
The swimming pattern depends on activation of ionotropic glutamatergic and glycinergic receptors
An important source of excitation within the spinal locomotor network is glutamatergic release from excitatory interneurons. To test whether the swimming activity induced by electrical stimulation of descending inputs is mediated by activation of ionotropic glutamate receptor, specific NMDA and AMPA receptor antagonists were used. Blockade of NMDA receptors by the antagonist AP-5 (50 μm) blocked the generation of swimming activity, which was replaced with tonic activity in the motor nerve (data not shown). To further test the role of NMDA receptors in the generation of swimming activity, we used the activity-dependent antagonist MK-801, which blocks only open NMDA channels. Unlike during AP-5 application, stimulation of descending inputs in MK-801 (10 μm) was still able to induce swimming activity, although the duration of the episode was much shorter (control: 35.8 ± 11.1 s; MK-801: 11.1 ± 4.5 s; p < 0.05; n = 6) (Fig. 6A). In addition, the peak burst frequency decreased from 6.5 ± 0.8 to 4.2 ± 0.9 Hz (p < 0.05; n = 6) (Fig. 6C), as did the amplitude of the bursts that deceased to 42.3 ± 9.6% of control (p < 0.01; n = 6) (Fig. 6A).
Figure 6.
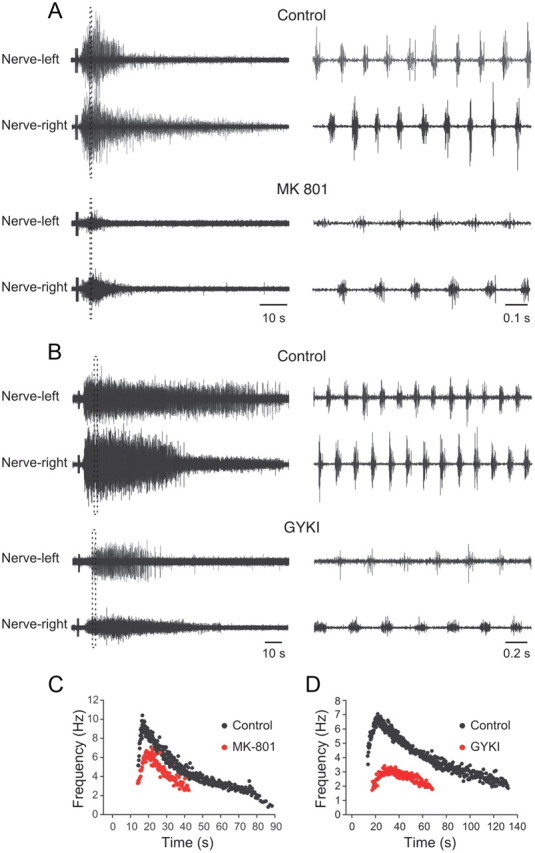
Contribution of ionotropic glutamate receptors to the expression of swimming activity. A, Application of the NMDA receptor antagonist MK-801 (10 μm) decreases the amplitude and frequency of the swimming bursts. B, Blockade of AMPA receptors with the antagonist GYKI 52466 (30 μm) also decreases the amplitude and frequency of the swimming bursts. C, Graph showing the instantaneous burst frequency during one episode in control and in MK-801. D, Graph showing the instantaneous burst frequency during one episode in control and GYKI 52466.
The contribution of AMPA receptors to the generation of swimming activity was also examined. Application of the AMPA receptor antagonist GYKI 52466 (30 μm) reversibly decreased the duration of the swimming episode from 85.4 ± 19.8 to 34.5 ± 4.8 s (p < 0.05; n = 6) (Fig. 6B,D). This was accompanied by a reversible decrease in the burst frequency from 5.4 ± 0.7 to 3.0 ± 0.5 Hz (p < 0.05; n = 6) (Fig. 6D), and in amplitude to 22.2 ± 4.6% of control (p < 0.01; n = 6) (Fig. 6B). Together, these results show that the expression of the full swimming frequency and burst amplitude is dependent on activation of both NMDA and AMPA receptors that seem to be activated by release of glutamate from descending inputs and from excitatory interneurons within the spinal locomotor network.
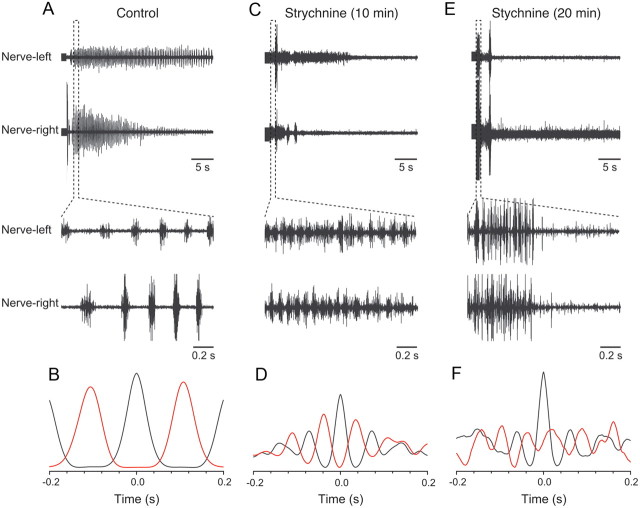
To determine whether inhibitory synaptic transmission is required to generate rhythmic activity or whether it is only responsible for maintaining the left-right alternation, the effect of the glycine receptor antagonist strychnine was tested. In control, swimming bouts were induced by the stimulation of the rostral spinal cord (Fig. 7A) and displayed coordinated left-right alternation (Fig. 7B). Application of strychnine (1 μm) resulted initially in an increase in the swimming burst frequency from 4.6 ± 0.6 to 12.6 ± 0.8 Hz (p < 0.01; n = 4) (Fig. 7C), while the left-right alternating pattern was still present (Fig. 7D). Prolonged strychnine application, however, affected the left-right coordination (Fig. 7E). The sides became uncoupled with no strict alternation of activity, although each side was still rhythmically active (Fig. 7F). These results suggest that glycinergic inhibition is important for left-right coordination and for setting the burst frequency, but it does not seem to contribute to the generation of rhythmic activity on each side of the spinal cord.
Figure 7.
Glycinergic synaptic transmission controls the speed and alternation of the swimming activity. A, Swimming activity is induced by electrical stimulation. B, Graph showing cross-correlation between the activity of left and right motor nerves. C, Application of strychnine (1 μm) initially increases the swimming burst frequency. D, Graph showing cross-correlation of the activity of left and right motor nerves that displays alternation. E, Prolonged application of strychnine decreases the episode duration and disrupts the left-right alternation. F, There is no strict alternation between the activity on the left and right motor nerves as shown by the lack of cross-correlation.
Activity of motoneurons during stimulation- and NMDA-induced swimming
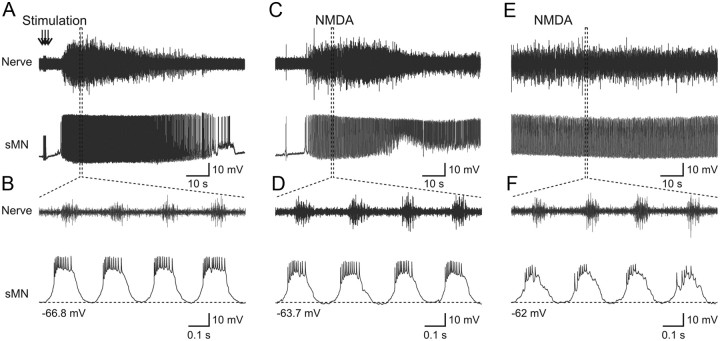
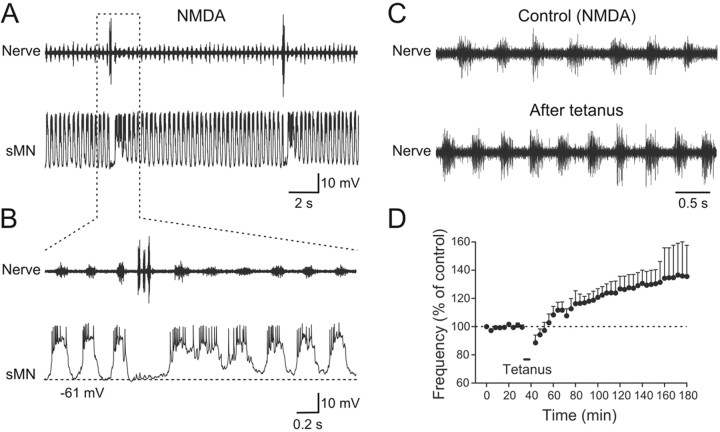
In most vertebrate preparations used to study locomotion, NMDA is used alone or in combination with other modulatory transmitters to induce locomotor activity. We therefore sought to compare the burst frequency and the activity of identified motoneurons (n = 8) during locomotion induced by electrical stimulation and by NMDA. Stimulation of descending inputs induced a long episode of swimming bursts in the motor nerve associated with membrane potential oscillation in the recorded secondary motoneuron (sMN) (Fig. 8A). Action potentials occur during the plateau depolarization of the sMN and had constant amplitude (Fig. 8B). At the onset of NMDA (40 μm), application swimming activity was induced and the membrane potential oscillations and firing of the sMN (Fig. 8C,D) resembled those seen during stimulation-induced swimming. When the swimming frequency reached steady state (ranging from 4 to 6 Hz) (Gabriel et al., 2008), the membrane potential of the recorded sMN was slightly depolarized, and the shape of the membrane oscillations changed compared with those occurring at the onset of NMDA application (Fig. 8E). The action potentials had occurred already during the rising phase of the oscillation, and their amplitude was shunted by the depolarization of the membrane potential (Fig. 8F). The membrane potential oscillations recorded in motoneurons during stimulation- and NMDA-induced swimming activity are the results of on-cycle excitation alternating with mid-cycle inhibition (Gabriel et al., 2009). Thus, while the frequency of locomotor activity induced by electrical stimulation and NMDA application was comparable, the shape of the membrane oscillations and action potentials were clearly affected by the prolonged NMDA application.
Figure 8.
Comparison of the activity of motoneurons during swimming induced by electrical stimulation and by NMDA. A, During swimming activity induced by electrical stimulation, the recorded sMN fires continuously during the entire episode. B, The sMN receives large synaptic drive that brings it to firing threshold. C, At the beginning of NMDA application, swimming activity is induced and the sMN fires action potential. D, The shape of the synaptic drive is somewhat different in NMDA-induced swimming activity compared with that induced by electrical stimulation. E, Prolonged NMDA application that allows reaching steady-state burst frequency affects the activity of the recorded sMN. F, The amplitude of the synaptic drive and the action potentials is decreased. The action potentials occur during the rising phase of the synaptic drive, not during the plateau depolarization as seen during stimulation-induced swimming activity (B).
In some preparations, NMDA application not only induced swimming but it also elicited spontaneous escape activity. As in the freely moving animals (Fig. 4), the escape consisted of a few bursts of large amplitude that interrupted the swimming activity (Fig. 9A). Some sMNs that were recruited during swimming became derecruited during escape (Fig. 9B). These results suggest that swimming and escape are generated by independent networks operating at different frequencies.
Figure 9.
Spontaneous switch between swimming and escape activity and plasticity of the locomotor circuitry. A, During swimming activity induced by NMDA, spontaneous episodes of escape occur with high burst frequency. B, During escape, the swimming activity and the firing of the recorded sMN are interrupted. C, Stimulation of the descending inputs induces an increase of the burst frequency of the locomotor rhythm induced by NMDA. In these experiments, the recording chamber was divided into two pools. Synaptic transmission was blocked in the rostral pool with a Ca2+-free solution containing AP-5 and GYKI 52466. Locomotor activity is induced in the caudal pool by application of NMDA. D, Graph showing the long-term increase in the burst frequency induced by stimulation of the descending inputs.
Long-term potentiation of the activity of the spinal locomotor network
Since the stimulation of descending inputs can activate the spinal locomotor network to elicit swimming activity, we then asked whether such stimulation can further boost the ongoing activity. For this, we used a double-pool recording chamber with the hindbrain and rostral spinal cord being perfused with Ca2+-free solution containing AP-5 and GYKI, while locomotor activity was induced by application of NMDA in the caudal spinal cord (Fig. 5A). Application of NMDA induced locomotor activity with constant burst frequency and amplitude (Fig. 9C). A brief stimulation of descending inputs (3× 1 s at 40 Hz) resulted in an increase in the burst frequency and amplitude (Fig. 9C). The increase in the burst frequency (25.7 ± 9.3%; n = 5) was long lasting because it persisted for >2 h after the end of the stimulation (Fig. 9D). These results show that in addition to being able to initiate the activity of the locomotor network, stimulation of descending inputs can induce long-term potentiation of the activity of the locomotor network.
Discussion
Motor behaviors are driven by dedicated neural networks that control the timing and the sequence of muscle contractions. Circuits in the spinal cord generate locomotor movements and integrate sensory inputs as well as inputs from supraspinal centers (Hultborn et al., 1998; Kiehn, 2006; Rossignol et al., 2006; Fetcho et al., 2008; Grillner and Jessell, 2009; El Manira and Kyriakatos, 2010). Unraveling the intrinsic function of the spinal network requires model systems that are accessible for the combination of molecular and genetic tools together with electrophysiology. In this regard, the zebrafish at early developmental stages has emerged as an attractive system that already enabled some of the neuronal components for swimming and escape behavior (Drapeau et al., 2002; Kimura et al., 2006; McLean et al., 2007; Fetcho et al., 2008; Liao and Fetcho, 2008; McLean et al., 2008; Satou et al., 2009; Fetcho and McLean, 2010). These studies need to be extended from larval to juvenile and adult stages to determine whether there is further refinement of the neuronal components of the locomotor circuit that is associated with a change in the swimming behavior, from larval burst swimming to the adult pattern of slow, steady swimming movements and the organization of the motor column (van Raamsdonk et al., 1982, 1983; Müller et al., 2000; Müller and van Leeuwen, 2004). Equally important is how the neural mechanisms responsible for interactions between the swimming and escape circuits are adapted as the animal's shape and speed of locomotion changes during development. To be able to use the adult brainstem-spinal cord preparation to tackle these questions, swimming activity needs to be activated by stimulating descending inputs that represent the natural source of excitatory drive to the spinal locomotor circuits. In this study, we show that coordinated swimming activity is induced by stimulation of a defined region at the first segments of the spinal cord. The burst amplitude and frequency range as well as the episode duration can increase by changing frequency and strength of the stimulus pulses. Another important finding of this study is the fact that stimulation-induced swimming displays a wide range of frequencies, while with NMDA the frequency is “locked” to one value for each concentration and with a smaller variation with increased concentrations (Gabriel et al., 2008). The frequencies obtained with stimulation are comparable to those recorded in freely swimming animals. During NMDA-induced swimming activity, additional input from descending fibers produces long-term potentiation of the burst frequency (Kettunen et al., 2005; Kyriakatos and El Manira, 2007; El Manira and Kyriakatos, 2010). The experimental approach used to induce fictive swimming in vitro bears similarities with the way the spinal locomotor networks are activated in vivo and are therefore more physiological than the use of pharmacology.
Zebrafish display different motor behaviors; kinematic analysis in larvae has defined different patterns of coordination of body axis and pectoral fins (Budick and O'Malley, 2000; Thorsen et al., 2004). These different repertoires of motor patterns are thought to result from discrete and interacting circuits that can be selectively activated by descending inputs from the brain. Studies in larval zebrafish have begun to explore the role of different interneuron types during escape and swimming as well as their activation by descending neurons (e.g., Mauthner cells) (Gahtan et al., 2002; Liao and Fetcho, 2008; Satou et al., 2009). Recently, it has been shown that there is a topographic order of recruitment of interneurons and motoneurons that match their order of development (McLean et al., 2007, 2008; El Manira and Grillner, 2008; McLean and Fetcho, 2009; Roberts et al., 2010). As zebrafish grow toward adulthood, their body shape changes, and the pattern and speed of locomotion is adapted; with larger body dimension, the swimming frequency decreases. We recently showed that the mechanisms controlling the recruitment of motoneurons are changing during maturation. In juvenile/adult zebrafish, motoneurons that are responsible for slow swimming display specific membrane properties and receive larger synaptic drive, but their order of recruitment is not correlated with their input resistance as shown in larvae (Gabriel et al., 2011). The fact that the juvenile/adult zebrafish in vitro preparation can sometimes display both swimming activity and escape will further allow for examination of the mechanisms of recruitment of the neurons involved and whether they are dedicated to one or shared between different motor circuits (Svoboda and Fetcho, 1996). While the activity of motoneurons recruited at slow swimming frequency seems to be interrupted during escape, the primary and dorsal secondary motoneurons, thought to mediate escape, continue to receive phasic synaptic inputs during swimming (Gabriel et al., 2008, 2009, 2011). In addition, it is possible that different interneurons are also taking part in the initiation, maintenance, and termination of the swimming episode. These subtle changes in neuronal recruitment are impossible to be accounted for with traditional pharmacological activation of the locomotor network. For example, it has been shown that different sets of interneurons are recruited at different swimming speeds (Kimura et al., 2006; McLean et al., 2008; McLean and Fetcho, 2009), such a pattern of recruitment will not be possible to detect during swimming with a constant frequency induced with NMDA.
In many preparations, locomotor activity can be initiated from specific supraspinal centers in the mesencephalon and diencephalon (Jordan et al., 1992; Fetcho and Svoboda, 1993; El Manira et al., 1997; Sirota et al., 2000; Cabelguen et al., 2003). Inputs from these regions converge on reticulospinal neurons, which in turn activate spinal networks (El Manira et al., 1997; Dubuc et al., 2008; Shaw et al., 2010). However, in all these cases, the expression of locomotor activity required that these centers are stimulated continuously and the activity declined rapidly upon termination of the stimulation. The nature of the brainstem commands to the spinal network has recently been re-evaluated in Xenopus tadpoles by recording hindbrain excitatory neurons and correlating their activity to the locomotor rhythm (Li et al., 2006, 2010; Soffe et al., 2009). It has been proposed that the descending rhythmic excitation from reticulospinal neurons in the caudal hindbrain imposes the basic swimming rhythm on the spinal circuitry. These hindbrain neurons have thus been considered as part of the rhythm-generating circuitry rather than simply supplying a tonic drive that is converted to a rhythmic pattern (Roberts et al., 2010). An important finding of our study is that in juvenile/adult zebrafish a brief input from descending fibers is turned into long-lasting coordinated swimming activity. This activity persists even after blockade of chemical synaptic transmission in the hindbrain and rostral spinal cord, suggesting that it is not driven by activity of a network of descending excitatory neurons. In the present study, we focused on defining a region that reproducibly induced swimming activity and did not attempt to define the types of tracts stimulated or their origin.
Like other vertebrate preparations, locomotor activity in zebrafish can be induced pharmacologically by activation of NMDA receptors (Cazalets et al., 1992; Zhang et al., 1996; Grillner, 2003; Cowley et al., 2005; Kiehn, 2006; McDearmid and Drapeau, 2006; Issberner and Sillar, 2007; Li et al., 2010; Talpalar and Kiehn, 2010). However, the NMDA-induced locomotor activity shows less frequency variation and therefore does not allow examining the activity of individual spinal network neurons over a large range of frequencies. In addition, the constant activation of NMDA receptors induces a tonic depolarization of the membrane potential of neurons that affects significantly the waveform of their membrane potential oscillations. Thus, the use of electrical stimulation to activate the spinal locomotor circuit has many advantages over pharmacological tools because the activity can be elicited repeatedly with a larger span of frequencies and recruited motor units.
In conclusion, we defined a region that reproducibly induced swimming activity and did not attempt to define the types of tracts stimulated or their origin. To understand the neural bases of motor behavior, it is important to determine not only during the mechanisms of its execution but also those controlling its initiation and termination. The results presented in this study provide a characterization of how spinal locomotor circuits are turned on by descending excitatory inputs and some initial insights into the interactions between swimming and escape circuits. Thus, this new information provides the basis for future characterization of the interneurons involved and the mechanisms setting their recruitment threshold during different motor behaviors.
Footnotes
This work was supported by the Swedish Research Council, the European Commission (Health-F2–2007-201144), the Karolinska Institutet, and the DFG (Bu857/7-1). J.A. received postdoctoral fellowships from the German Science Foundation. We thank Drs. Sten Grillner and Russell Hill for comments on the manuscript. We are grateful to Dr. Tim Mentel for his helping in setting up the in vivo preparation and EMG recordings.
Reference
- Berkowitz A, Roberts A, Soffe SR. Roles for multifunctional and specialized spinal interneurons during motor pattern generation in tadpoles, zebrafish larvae, and turtles. Front Behav Neurosci. 2010;4:36. doi: 10.3389/fnbeh.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budick SA, O'Malley DM. Locomotor repertoire of the larval zebrafish: swimming, turning and prey capture. J Exp Biol. 2000;203:2565–2579. doi: 10.1242/jeb.203.17.2565. [DOI] [PubMed] [Google Scholar]
- Cabelguen JM, Bourcier-Lucas C, Dubuc R. Bimodal locomotion elicited by electrical stimulation of the midbrain in the salamander Notophthalmus viridescens. J Neurosci. 2003;23:2434–2439. doi: 10.1523/JNEUROSCI.23-06-02434.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol. 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong M, Drapeau P. Interaction between hindbrain and spinal networks during the development of locomotion in zebrafish. Dev Neurobiol. 2007;67:933–947. doi: 10.1002/dneu.20398. [DOI] [PubMed] [Google Scholar]
- Clarac F, Pearlstein E, Pflieger JF, Vinay L. The in vitro neonatal rat spinal cord preparation: a new insight into mammalian locomotor mechanisms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190:343–357. doi: 10.1007/s00359-004-0499-2. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Zaporozhets E, Maclean JN, Schmidt BJ. Is NMDA receptor activation essential for the production of locomotor-like activity in the neonatal rat spinal cord? J Neurophysiol. 2005;94:3805–3814. doi: 10.1152/jn.00016.2005. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Orlovsky GN. Encoding and decoding of reticulospinal commands. Brain Res Rev. 2002;40:166–177. doi: 10.1016/s0165-0173(02)00199-6. [DOI] [PubMed] [Google Scholar]
- Drapeau P, Saint-Amant L, Buss RR, Chong M, McDearmid JR, Brustein E. Development of the locomotor network in zebrafish. Prog Neurobiol. 2002;68:85–111. doi: 10.1016/s0301-0082(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Dubuc R, Brocard F, Antri M, Fénelon K, Gariépy JF, Smetana R, Ménard A, Le Ray D, Viana Di Prisco G, Pearlstein E, Sirota MG, Derjean D, St-Pierre M, Zielinski B, Auclair F, Veilleux D. Initiation of locomotion in lampreys. Brain Res Rev. 2008;57:172–182. doi: 10.1016/j.brainresrev.2007.07.016. [DOI] [PubMed] [Google Scholar]
- El Manira A, Clarac F. Presynaptic inhibition is mediated by histamine and GABA in the crustacean escape reaction. J Neurophysiol. 1994;71:1088–1095. doi: 10.1152/jn.1994.71.3.1088. [DOI] [PubMed] [Google Scholar]
- El Manira A, Grillner S. Switching gears in the spinal cord. Nat Neurosci. 2008;11:1367–1368. doi: 10.1038/nn1208-1367. [DOI] [PubMed] [Google Scholar]
- El Manira A, Kyriakatos A. The role of endocannabinoid signaling in motor control. Physiology. 2010;25:230–238. doi: 10.1152/physiol.00007.2010. [DOI] [PubMed] [Google Scholar]
- El Manira A, Pombal MA, Grillner S. Diencephalic projection to reticulospinal neurons involved in the initiation of locomotion in adult lampreys Lampetra fluviatilis. J Comp Neurol. 1997;389:603–616. [PubMed] [Google Scholar]
- Fetcho JR, McLean DL. Some principles of organization of spinal neurons underlying locomotion in zebrafish and their implications. Ann N Y Acad Sci. 2010;1198:94–104. doi: 10.1111/j.1749-6632.2010.05539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetcho JR, Svoboda KR. Fictive swimming elicited by electrical stimulation of the midbrain in goldfish. J Neurophysiol. 1993;70:765–780. doi: 10.1152/jn.1993.70.2.765. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, Higashijima S, McLean DL. Zebrafish and motor control over the last decade. Brain Res Rev. 2008;57:86–93. doi: 10.1016/j.brainresrev.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel JP, Mahmood R, Walter AM, Kyriakatos A, Hauptmann G, Calabrese RL, El Manira A. Locomotor pattern in the adult zebrafish spinal cord in vitro. J Neurophysiol. 2008;99:37–48. doi: 10.1152/jn.00785.2007. [DOI] [PubMed] [Google Scholar]
- Gabriel JP, Mahmood R, Kyriakatos A, Söll I, Hauptmann G, Calabrese RL, El Manira A. Serotonergic modulation of locomotion in zebrafish: endogenous release and synaptic mechanisms. J Neurosci. 2009;29:10387–10395. doi: 10.1523/JNEUROSCI.1978-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel JP, Ausborn J, Ampatzis K, Mahmood R, Eklöf-Ljunggren E, El Manira A. Principles governing recruitment of motoneurons during swimming in zebrafish. Nat Neurosci. 2011;14:93–99. doi: 10.1038/nn.2704. [DOI] [PubMed] [Google Scholar]
- Gahtan E, Sankrithi N, Campos JB, O'Malley DM. Evidence for a widespread brain stem escape network in larval zebrafish. J Neurophysiol. 2002;87:608–614. doi: 10.1152/jn.00596.2001. [DOI] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009;10:507–518. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19:572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Conway BA, Gossard JP, Brownstone R, Fedirchuk B, Schomburg ED, Enríquez-Denton M, Perreault MC. How do we approach the locomotor network in the mammalian spinal cord? Ann N Y Acad Sci. 1998;860:70–82. doi: 10.1111/j.1749-6632.1998.tb09039.x. [DOI] [PubMed] [Google Scholar]
- Issberner JP, Sillar KT. The contribution of the NMDA receptor glycine site to rhythm generation during fictive swimming in Xenopus laevis tadpoles. Eur J Neurosci. 2007;26:2556–2564. doi: 10.1111/j.1460-9568.2007.05892.x. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Brownstone RM, Noga BR. Control of functional systems in the brainstem and spinal cord. Curr Opin Neurobiol. 1992;2:794–801. doi: 10.1016/0959-4388(92)90136-9. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Rev. 2008;57:183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Kettunen P, Kyriakatos A, Hallén K, El Manira A. Neuromodulation via conditional release of endocannabinoids in the spinal locomotor network. Neuron. 2005;45:95–104. doi: 10.1016/j.neuron.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakatos A, El Manira A. Long-term plasticity of the spinal locomotor circuitry mediated by endocannabinoid and nitric oxide signaling. J Neurosci. 2007;27:12664–12674. doi: 10.1523/JNEUROSCI.3174-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBeau FE, El Manira A, Griller S. Tuning the network: modulation of neuronal microcircuits in the spinal cord and hippocampus. Trends Neurosci. 2005;28:552–561. doi: 10.1016/j.tins.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Liao JC, Fetcho JR. Shared versus specialized glycinergic spinal interneurons in axial motor circuits of larval zebrafish. J Neurosci. 2008;28:12982–12992. doi: 10.1523/JNEUROSCI.3330-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Soffe SR, Wolf E, Roberts A. Persistent responses to brief stimuli: feedback excitation among brainstem neurons. J Neurosci. 2006;26:4026–4035. doi: 10.1523/JNEUROSCI.4727-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, Roberts A, Soffe SR. Specific brainstem neurons switch each other into pacemaker mode to drive movement by activating NMDA receptors. J Neurosci. 2010;30:16609–16620. doi: 10.1523/JNEUROSCI.3695-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DW, Westerfield M. Function of identified motoneurones and co-ordination of primary and secondary motor systems during zebra fish swimming. J Physiol. 1988;403:73–89. doi: 10.1113/jphysiol.1988.sp017239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D, Schulz DJ, Taylor AL. Invertebrate central pattern generation moves along. Curr Biol. 2005;15:R685–R699. doi: 10.1016/j.cub.2005.08.022. [DOI] [PubMed] [Google Scholar]
- McDearmid JR, Drapeau P. Rhythmic motor activity evoked by NMDA in the spinal zebrafish larva. J Neurophysiol. 2006;95:401–417. doi: 10.1152/jn.00844.2005. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Spinal interneurons differentiate sequentially from those driving the fastest swimming movements in larval zebrafish to those driving the slowest ones. J Neurosci. 2009;29:13566–13577. doi: 10.1523/JNEUROSCI.3277-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean DL, Fan J, Higashijima S, Hale ME, Fetcho JR. A topographic map of recruitment in spinal cord. Nature. 2007;446:71–75. doi: 10.1038/nature05588. [DOI] [PubMed] [Google Scholar]
- McLean DL, Masino MA, Koh IY, Lindquist WB, Fetcho JR. Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci. 2008;11:1419–1429. doi: 10.1038/nn.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentel T, Krause A, Pabst M, El Manira A, Büschges A. Activity of dorsal fin muscles and motoneurons during locomotor activity in lamprey in vivo and in vitro. Eur J Neurosci. 2006;23:2012–2026. doi: 10.1111/j.1460-9568.2006.04738.x. [DOI] [PubMed] [Google Scholar]
- Müller UK, van Leeuwen JL. Swimming of larval zebrafish: ontogeny of body waves and implications for locomotory development. J Exp Biol. 2004;207:853–868. doi: 10.1242/jeb.00821. [DOI] [PubMed] [Google Scholar]
- Müller UK, Stamhuis EJ, Videler JJ. Hydrodynamics of unsteady fish swimming and the effects of body size: comparing the flow fields of fish larvae and adults. J Exp Biol. 2000;203:193–206. doi: 10.1242/jeb.203.2.193. [DOI] [PubMed] [Google Scholar]
- Roberts A, Li WC, Soffe SR, Wolf E. Origin of excitatory drive to a spinal locomotor network. Brain Res Rev. 2008;57:22–28. doi: 10.1016/j.brainresrev.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Roberts A, Li WC, Soffe SR. How neurons generate behavior in a hatchling amphibian tadpole: an outline. Front Behav Neurosci. 2010;4:16. doi: 10.3389/fnbeh.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Satou C, Kimura Y, Kohashi T, Horikawa K, Takeda H, Oda Y, Higashijima S. Functional role of a specialized class of spinal commissural inhibitory neurons during fast escapes in zebrafish. J Neurosci. 2009;29:6780–6793. doi: 10.1523/JNEUROSCI.0801-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt BJ, Hochman S, MacLean JN. NMDA receptor-mediated oscillatory properties: potential role in rhythm generation in the mammalian spinal cord. Ann N Y Acad Sci. 1998;860:189–202. doi: 10.1111/j.1749-6632.1998.tb09049.x. [DOI] [PubMed] [Google Scholar]
- Shaw AC, Jackson AW, Holmes T, Thurman S, Davis GR, McClellan AD. Descending brain neurons in larval lamprey: spinal projection patterns and initiation of locomotion. Exp Neurol. 2010;224:527–541. doi: 10.1016/j.expneurol.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota MG, Di Prisco GV, Dubuc R. Stimulation of the mesencephalic locomotor region elicits controlled swimming in semi-intact lampreys. Eur J Neurosci. 2000;12:4081–4092. doi: 10.1046/j.1460-9568.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- Soffe SR, Roberts A, Li WC. Defining the excitatory neurons that drive the locomotor rhythm in a simple vertebrate: insights into the origin of reticulospinal control. J Physiol. 2009;587:4829–4844. doi: 10.1113/jphysiol.2009.175208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda KR, Fetcho JR. Interactions between the neural networks for escape and swimming in goldfish. J Neurosci. 1996;16:843–852. doi: 10.1523/JNEUROSCI.16-02-00843.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccola G, Nistri A. Oscillatory circuits underlying locomotor networks in the rat spinal cord. Crit Rev Neurobiol. 2006;18:25–36. doi: 10.1615/critrevneurobiol.v18.i1-2.40. [DOI] [PubMed] [Google Scholar]
- Talpalar AE, Kiehn O. Glutamatergic mechanisms for speed control and network operation in the rodent locomotor CpG. Front Neural Circuits. 2010;4:pii, 19. doi: 10.3389/fncir.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen DH, Cassidy JJ, Hale ME. Swimming of larval zebrafish: fin-axis coordination and implications for function and neural control. J Exp Biol. 2004;207:4175–4183. doi: 10.1242/jeb.01285. [DOI] [PubMed] [Google Scholar]
- van Raamsdonk W, van't Veer L, Veeken K, Heyting C, Pool CW. Differentiation of muscle fiber types in the teleost Brachydanio rerio, the zebrafish. Posthatching development. Anat Embryol (Berl) 1982;164:51–62. doi: 10.1007/BF00301878. [DOI] [PubMed] [Google Scholar]
- van Raamsdonk W, Mos W, Smit-Onel MJ, van der Laarse WJ, Fehres R. The development of the spinal motor column in relation to the myotomal muscle fibers in the zebrafish (Brachydanio rerio). I. Posthatching development. Anat Embryol (Berl) 1983;167:125–139. doi: 10.1007/BF00304606. [DOI] [PubMed] [Google Scholar]
- Zhang W, Pombal MA, El Manira A, Grillner S. Rostrocaudal distribution of 5-HT innervation in the lamprey spinal cord and differential effects of 5-HT on fictive locomotion. J Comp Neurol. 1996;374:278–290. doi: 10.1002/(SICI)1096-9861(19961014)374:2<278::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]